Natural and Anthropogenic Particulate-Bound Aliphatic And
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

2010 Year Book
2010 YEAR BOOK www.massbaysailing.org $5.00 HILL & LOWDEN, INC. YACHT SALES & BROKERAGE J boat dealer for Massachusetts and southern new hampshire Hill & Lowden, Inc. offers the full range of new J Boat performance sailing yachts. We also have numerous pre-owned brokerage listings, including quality cruising sailboats, racing sailboats, and a variety of powerboats ranging from runabouts to luxury cabin cruisers. Whether you are a sailor or power boater, we will help you find the boat of your dreams and/or expedite the sale of your current vessel. We look forward to working with you. HILL & LOWDEN, INC. IS CONTINUOUSLY SEEKING PRE-OWNED YACHT LISTINGS. GIVE US A CALL SO WE CAN DISCUSS THE SALE OF YOUR BOAT www.Hilllowden.com 6 Cliff Street, Marblehead, MA 01945 Phone: 781-631-3313 Fax: 781-631-3533 Table of Contents ______________________________________________________________________ INFORMATION Letter to Skippers ……………………………………………………. 1 2009 Offshore Racing Schedule ……………………………………………………. 2 2009 Officers and Executive Committee …………… ……………............... 3 2009 Mass Bay Sailing Delegates …………………………………………………. 4 Event Sponsoring Organizations ………………………………………................... 5 2009 Season Championship ………………………………………………………. 6 2009 Pursuit race Championship ……………………………………………………. 7 Salem Bay PHRF Grand Slam Series …………………………………………….. 8 PHRF Marblehead Qualifiers ……………………………………………………….. 9 2009 J105 Mass Bay Championship Series ………………………………………… 10 PHRF EVENTS Constitution YC Wednesday Evening Races ……………………………………….. 11 BYC Wednesday Evening -

Proposed Revisions to 314 CMR 4.00 (Tables and Figures, Clean)
Please see the 314 CMR 4.00 Summary and Notice to Reviewers document, as well as the Fact Sheets on particular topics for additional information and explanatory detail associated with these proposed regulatory changes. These documents are available on the MassDEP Website. 314 CMR: DIVISION OF WATER POLLUTION CONTROL 4.06: continued LIST OF TABLES AND FIGURES* TABLE & TABLE AND CORRESPONDING FIGURE TITLE Page # FIGURE # A (Figure only) River Basins and Coastal Drainage Areas TF-2 1 Blackstone River Basin TF-3 2 Boston Harbor Drainage Area (formerly Boston Harbor Drainage System and Mystic, Neponset and Weymouth & Weir River Basins) TF-8 3 Buzzards Bay Coastal Drainage Area TF-17 4 Cape Cod Coastal Drainage Area TF-22 5 Charles River Basin TF-30 6 Chicopee River Basin TF-34 7 Connecticut River Basin TF-40 8 Deerfield River Basin TF-49 9 Farmington River Basin TF-58 10 French River Basin TF-60 11 Housatonic River Basin TF-62 12 Hudson River Basin (formerly Hoosic, Kinderhook and Bashbish) TF-70 13 Ipswich River Basin TF-76 14 Islands Coastal Drainage Area (formerly Martha's Vineyard and Nantucket) TF-79 15 Merrimack River Basin TF-81 16 Millers River Basin TF-86 17 Narragansett Bay and Mount Hope Bay Drainage Area TF-90 18 Nashua River Basin TF-93 19 North Coastal Drainage Area TF-103 20 Parker River Basin TF-109 21 Quinebaug River Basin TF-113 22 Shawsheen River Basin TF-116 23 South Coastal Drainage Area TF-118 24 Sudbury, Assabet, and Concord (SuAsCo) River Basin (formerly Concord) TF-123 25 Taunton River Basin TF-128 26 Ten Mile River Basin TF-132 27 Westfield River Basin TF-134 28 (Table only) Site-Specific Criteria TF-144 29 (Table only) GenerallyApplicable Criteria: 29a. -
CPB1 C10 WEB.Pdf
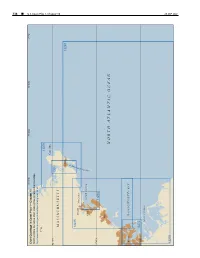
338 ¢ U.S. Coast Pilot 1, Chapter 10 Chapter 1, Pilot Coast U.S. 70°45'W 70°30'W 70°15'W 71°W Chart Coverage in Coast Pilot 1—Chapter 10 NOAA’s Online Interactive Chart Catalog has complete chart coverage http://www.charts.noaa.gov/InteractiveCatalog/nrnc.shtml 71°W 13279 Cape Ann 42°40'N 13281 MASSACHUSETTS Gloucester 13267 R O B R A 13275 H Beverly R Manchester E T S E C SALEM SOUND U O Salem L G 42°30'N 13276 Lynn NORTH ATLANTIC OCEAN Boston MASSACHUSETTS BAY 42°20'N 13272 BOSTON HARBOR 26 SEP2021 13270 26 SEP 2021 U.S. Coast Pilot 1, Chapter 10 ¢ 339 Cape Ann to Boston Harbor, Massachusetts (1) This chapter describes the Massachusetts coast along and 234 miles from New York. The entrance is marked on the northwestern shore of Massachusetts Bay from Cape its eastern side by Eastern Point Light. There is an outer Ann southwestward to but not including Boston Harbor. and inner harbor, the former having depths generally of The harbors of Gloucester, Manchester, Beverly, Salem, 18 to 52 feet and the latter, depths of 15 to 24 feet. Marblehead, Swampscott and Lynn are discussed as are (11) Gloucester Inner Harbor limits begin at a line most of the islands and dangers off the entrances to these between Black Rock Danger Daybeacon and Fort Point. harbors. (12) Gloucester is a city of great historical interest, the (2) first permanent settlement having been established in COLREGS Demarcation Lines 1623. The city limits cover the greater part of Cape Ann (3) The lines established for this part of the coast are and part of the mainland as far west as Magnolia Harbor. -

Air Bubbles the Newsletter of the North Shore Frogmen’S Club Volume 54, Number 6 June 2012
Air Bubbles The Newsletter of the North Shore Frogmen’s Club Volume 54, Number 6 June 2012 Presidents’ Messages - June 2012 Coming Club Events It's beginning to feel a lot like dive season! Now that it's June, summer diving is beginning to get into full swing, especially with May 31: Barbara Warren of a balmy water temp of upper 40s to lower 50s at depth! You Salem Sound CoastWatch – will don't even need the dry suit anymore, though some may disagree. give a presentation on local We have a busy month ahead. June 7th Mike Walsh will be marine invasive species. presenting on marine organism camouflage and Indonesia and June 28th is our first beach meeting at White Beach, Manchester. June 7: Mike Walsh will give a And, hopefully plenty of diving in between! presentation on Marine Organism This month I'd like to recognize Graham Smith as Diver of the Camouflage and Indonesia Month for frequently being at the BK parking lot on Sunday mornings to go diving. And for Member of the Month, Mary June 28: Beach Meeting at White Howard for always kindly reminding me that the President's Beach in Manchester by the Sea message is overdue. Thanks for your patience. Happy Diving! Meg! Diver of the Month for June 2012 Please Welcome New Member Graham Smith Seth Rakiey for frequently being at the BK parking lot on Sunday mornings to go diving From Grand Cayman, voted in as newest member on May 24. Member of the Month In this Issue: NOTICE for June 2012 Dues for 2012 President’s Message pg. -

Ns Newsletter 2010-0
Covering Aids to Navigation, Bridges, & Chart Updating Activity Accuracy - Credibility – Professionalism - Service to the Coast Guard & NOAA-NOS The 2010 Coast Guard Light List: The new light list is now available from the Coast Guard Navigation Center web site and all other copies of the light list are now obsolete and should not be used. http://www.navcen.uscg.gov The 2010 Light List for the First Coast Guard District is available by just clicking on the map that will appear. The publication contains a list of lights, sound signals, buoys, daybeacons, and other aids to navigation. This publication is in PDF format. The Light List is not corrected on line so it may make sense to save it to your hard-drive. Print only the pages that you need only when you need them while verifying PATONs on scene. Each aid to navigation should be checked against the latest copy of the Light List as a standard procedural part of a PATON’s verification. Active AVs-Aid Verifiers are encouraged to download and print out that section of the Light List that covers the PA-Patrol Area(s) where that plan to work on 2010. You are required to validate your observation of each PATON to the data shown in the Light List and report any discrepancies. SPECIAL NOTICE: DUE TO THE TERMINATION OF UNITED STATES LORAN-C, INFORMATION PERTAINING TO LORAN-C HAS BEEN REMOVED FROM THE ELECTRONIC VERSIONS OF THE 2010 LIGHT LIST BOOKS. THE UPDATED VESION IS LOCATED ON USCG NAVIGATION CENTER WEBSITE AT: http://navcen.uscg.gov/pubs/LightLists/LightLists.htm COAST GUARD DISTRICT 1 LOCAL NOTICE TO MARINERS: All Aid Verifiers, and all operational members should also be receiving a copy of the Local Notice to Mariners via email from U.S. -

(DMMP) Draft Environmental Impact Report (DEIR) for Salem Harbor
REVIEW DRAFT 10/15/99 DREDGE MATERIAL MANAGEMENT PLAN (DMMP) DRAFT ENVIRONMENTAL IMPACT REPORT (DEIR) FOR SALEM HARBOR MASSACHUSETTS OFFICE OF COASTAL ZONE MANAGEMENT (CZM) PREPARED BY: MAGUIRE GROUP INC. GROUP REVIEW DRAFT 10/15/99 DREDGE MATERIAL MANAGEMENT PLAN (DMMP) DRAFT ENVIRONMENTAL IMPACT REPORT (DEIR) FOR SALEM HARBOR DMMP CONSULTANT TEAM: Maguire Group Inc. The BSC Group CoastalVision GEi Consultants SAIC REVIEW DRAFT - 10/15/99 TABLE OF CONTENTS Secretary's Certificate on the Salem Harbor DMMP 1.0 EXECUTIVE SUMMARY .................................................... 1 - 1 2.0 INTRODUCTION .......................................................... 2 - 1 2.1 Background of the MA CZM DMMP ................................... 2 - 1 2.2 Massachusetts Environmental Policy Act (MEP A) Procedural History ....... 2 - 4 2.3 Scoping and Coordination Summary .................................... 2 - 4 2.3.1 Coordination with Port Planning Process ............................ 2 - 5 2.3.2 Coordination with Federal Agencies ................................ 2 - 9 3.0 PURPOSE AND NEED ..................................................... 3 - 1 3.1 Project Purpose ..................................................... 3 - 1 3.1.1 Basic Project Purpose .......................................... 3 - 1 3.2 Port Planning Context ................................................ 3 - 2 3.3 Sediment Quality and Quantity ........................................ 3 - 5 3.3.1 Dredging Inventory ............................................ 3 - 5 3.3.2 Sediment -

Mitb78001 Full.Pdf
~ ~ ~ e I- - ~ ~ p Q I 92! 17 .rr~ tb~ 4 I hl m J mnrr lg ~ Agniyrrnm,"-' ' ! !! e ~ t ~ ~ ~ ~ I p I I- - ~ ~ Lynn Harbor: Planning For Coastal Development Report Based on Interdepartmental Student Projects In Systems Engineering at the Massachusetts Institute of Technology Edited by I,isa Rosenbaua With a Poreword by William M. Seifert B.I.T. Sea Grant Prograa massachusetts Institute af Technology Cambridge, ilassachusetts This report describes the results of research done as part of the 5.I T Sea Grant Program with support from the Office of Sea Grant in the National Oceanic and Atmospheric Admini- stration, United States Department of Ccmmerce, through grant number 04-6-158-44081, and from the massachusetts Institute of Technology. The United States government is authorized to produce and distribute reprints for governmental purposes notwithstanding any copyright notation that may appear hereon. Lynn Harbor: Planning for Coastal Development Report Number 5ITSG 78-3 Index Number 77-103Udp Say 1978 hassachusetts Institute of Technology Cambridge, massachusetts 02139 ~ ~ ~ h ~ I T. SEA GRANT PUBLICATIONS Related Reports hcpherson, Roy Nick, ed., Gloucester Resource Stu~d. hITSG 74-3, Caahridge: hassachusetts Institute of Technolcgy, Noveaber 1973, 179 pp. 45.00. Zngellenner, Thoaas, Fred Curtis, and Millias Seifert, eds., The Boston South Shore Area: Some Problems and Conflicts. 8ETSG 75-23. Canhridge: hassachusetts Institute of Technology, August 1975. 175 pp. 45.00 Herr, philip, ee., ~haaa in Gloacesteris Coast. hITSG 77-23. Caahridge: hassachusetts Institute of Technology, Novesber 1977- 67 pp- $2.50 The Sea Grant Inforaation Center naintains an inventory of technical publications. -

CDSOA Cape Dory Float-In
CDSOA Cape Cod Loop Cruise Cape Dorys and the Historic Harbors of Cape Cod August 15-19, 2005 Questions? Contact Fleet Captain Catherine Monaghan Ph: 732-381-3549; Email: [email protected] Monday, 8/15--Provincetown, MA Figure 1. Provincetown Harbor, MA Fleet communications will be on VHF Ch. 16 and 68. Plan on meeting in the anchorage of Provincetown Harbor in the afternoon on Monday, August 15th where Cruise Captains Cathy Monaghan and Bruce Halley will be waiting aboard their Cape Dory 32 Realization for your arrival. Anchor just outside the mooring field southwest of the breakwater or reserve a mooring from the Provincetown Marina (508-487-0571, VHF Ch. 9) or Provincetown Mooring Services (VHF Ch. 9). Entering Provincetown Harbor is easy as the harbor is wide and deep. If you're coming from the outside, give Race Point a wide berth. From any direction your first mark will then be Fl G 4s BELL G"1" south of Wood End. Head for Q G BELL G"3" east of Long Point, leaving it to port before heading into the harbor. The breakwater is approximately 26.1 nm from the entrance to Plymouth Bay, approximately 21.3 nm from QG BELL G"3" at the entrance to the Cape Cod Canal, and 27 nm from the Wellfleet breakwater light Fl R 2.5s 16ft 5M "14". CDSOA, Inc. - 2 - Effective: 6 May 2005 Use tide tables for Boston. High tide in Provincetown is 16 minutes later and low tide is 18 minutes later. Mean tidal range is about 9 feet. -

Hill & Lowden, Inc
SPONSOR OF THE YEARBOOK 2015 www.massbaysailing.org HILL & LOWDEN, INC. YACHT SALES & BROKERAGE J/Boats Dealer for Massachusetts and Southern New Hampshire J/88 – Friendly Family Speedster J/111 – Fast, One-Design 36 footer Hill & Lowden, Inc. offers the full range of new J/Boats performance sailing yachts. We also have numerous pre-owned brokerage listings, including quality cruising and racing sailboats, and a variety of powerboats ranging from runabouts to luxury cabin cruisers. Whether you are a sailor or power boater, we will help you find the boat of your dreams and/or expedite the sales of you current vessel. We look forward to working with you. J/70 – New trailerable one-design A variety of motor yachts HILL & LOWDEN, INC. IS CONTINUOUSLY SEEKING PRE-OWNED YACHT LISTINGS. GIVE US A CALL SO WE CAN DISCUSS THE SALE OF YOUR BOAT. www.HillLowden.com 6 Cliff Street, Marblehead, MA 01945 Phone: (781) 631-3313 Fax: (781) 631-3533 TABLE OF CONTENTS 2015 INFORMATION Welcome Letter ………..……………………….……………………………. 2 Officers and Executive Committee ……………………….……….............. 4 Sailing Delegates ………………..…………………………..………………. 5 Sponsoring Organizations …………..…..……………..……….................. 6 US SAILING Judges - Massachusetts …………………………………..… 8 General Sailing Instructions ………………….….…………………………. 11 Season Scoring System & Class Splits ……..……..…………….……….. 14 Standard Courses …………………..…….…………….……….................. 15 Standard Rounding Marks ………………………….………………………. 16 Offshore Racing Schedule ………………………………..…………………. 18 Gulf of Maine Ocean Racing Events -

Proposed Revisions to 314 CMR 4.00 (Tables and Figures, Redline)
Please see the 314 CMR 4.00 Summary and Notice to Reviewers document, as well as the Fact Sheets on particular topics for additional information and explanatory detail associated with these proposed regulatory changes. These documents are available on the MassDEP Website. 314 CMR: DIVISION OF WATER POLLUTION CONTROL 4.06: continued LIST OF TABLES AND FIGURES* TABLES & TABLE AND CORRESPONDING FIGURE TITLE Page # FIGURE # A (Figure only) River Basins and Coastal Drainage Areas TF-2 1 Blackstone River Basin Hudson River Basin (formerly Hoosic) TF-3 2 Boston Harbor Drainage Area (formerly Boston Harbor Drainage System and Mystic, Neponset and Weymouth & Weir River Basins) TF-8 Housatonic River Basin 2 Kinderhook and Bashbish River Basins) 3 Buzzards Bay Coastal Drainage Area Farmington River Basin TF-17 4 Cape Cod Coastal Drainage Area Westfield River Basin TF-22 5 Charles River Basin Deerfield River Basin TF-30 6 Chicopee River Basin Connecticut River Basin TF-34 7 Connecticut River Basin Millers River Basin TF-40 8 Deerfield River Basin Chicopee River Basin TF-49 9 Farmington River Basin Quinebaug River Basin TF-58 10 French River BasinFrench River Basin TF-60 11 Housatonic River Basin Blackstone River Basin TF-62 12 Hudson River Basin (formerly Hoosic, Kinderhook and Bashbish) Ten Mile River Basin TF-70 13 Ipswich River Basin Narragansett Bay/Mount Hope Bay Drainage Area TF-76 14 Islands Coastal Drainage Area (formerly Martha's Vineyard and Nantucket) Taunton River Basin TF-79 15 Merrimack River Basin Boston Harbor Drainage Area (formerly -

Decimal Style Report
Feasibility Study of Constructing the New S-145 and T-146 Transmission Lines via a Horizontal Directional Drill Installation under the Salem Harbor prepared for New England Power Company d/b/a National Grid Waltham, MA July 2013 Project No. 53411 prepared by Burns & McDonnell Engineering Company, Inc. Kansas City, Missouri COPYRIGHT © 2013 BURNS & McDONNELL ENGINEERING COMPANY, INC. Feasibility Study of Constructing the New S-145 and T-146 Transmission Lines via a Horizontal Directional Drill Installation Under the Salem Harbor July 2013 Executive Summary EXECUTIVE SUMMARY Burns & McDonnell (BMcD) was requested to conduct an investigation into the feasibility and costs for installing the new S-145 and T-146 circuits under Salem Harbor via a Horizontal Directional Drill (HDD) for the purposes of avoiding a land installation through parts of Salem, MA. In support of this request, BMcD, along with their subconsultant Haley & Aldrich, Inc. (H&A), has prepared this Feasibility Study of Constructing the New S-145 and T-146 Transmission Lines via a Horizontal Directional Drill Installation Under the Salem Harbor. Please note the following clarifications: • This Feasibility Study is limited to the installation of electrical cables under Salem Harbor via HDD and does not reflect on other electrical cable or HDD projects. All other projects should be evaluated independently on their own merits. • This Feasibility Study does not compare the installation of the cables via HDD to other potential installation alternatives including underground or overhead cables. • This Feasibility Study does not identify, or include the cost of obtaining, the environmental, construction and zoning permits required for the installation of electrical cables under Salem Harbor via HDD, nor does it make a determination of the feasibility of acquiring said permits. -

B.A. Summa Cum Laude, University of Minnesota (1970) I
I DEVELOPING LYNN HARBOR: PROCESS, POLICIES, AITD IMPLEMEflTATION by CHARLES ALFRED KUBAT B.A. Summa Cum Laude, University of Minnesota (1970) B. Arch. High Honors, University of Minnesota (1970) Submitted in partic1 fulfillment of the requirements for the degree of MASTER OF CITY PLANNING at the MASSACHUSETTS INSTITUTE OF TECHNOLOGY May 1977 Signature of Author . .. I e, ' r u './.W="'1 . .. .. Department of Urban.jtudies and Planning, Iay 18, 1977 Certified by .... 0 . Thesis Supervisor Accepted by . wv .* . ., -. - . -0 -0 - - - --0 Chairman, Dep tm tal 'ntteeon Graduate Students II DEVELOPING LYNN HARBOR - PROCESS, POLICIES AND IIPLIEN4TATION by CHARLES ALFRED KUBAT Submitted to the Department of Urban Studies and Planning in May 1977 in partial fulfillment of the requirements for the degree of Master of City Planning. ABSTRACT This document is prepared as a report to the Lynn Departments of Community Development and Planning, and to the Lynn Port Authority as a basis for harbor area land use/economic development planning and policy recommendations to the mayor and city council. The recommendations for policy objectives and the city actions neces- sary to implement those objectives result from an analysis of community planning objectives, the regional context for development (development competition, transportation linkages, and coastal zone management poli- cies), the local context (job needs, tax base and market prospects), existing harbor conditions (land use, water use, image, ownership, utilities, etc.) and an assessment of the potential for development of activity types such as water related industry (seafood processing), water based recreation (marinas), and waterfront supportive uses (retail uses, etc.). Policy recommendations are made concerning the public approval process, utility infrastructure, land activities, water activities, "place" quality and movement.