Download Article (PDF)
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Konstantinov Simov 2018 Plumiger.Pdf
A peer-reviewed open-access journal ZooKeys 796:Review 215–239 of the (2018) subgenus Plumiger of Myrmecophyes, with description of a new species... 215 doi: 10.3897/zookeys.796.21877 RESEARCH ARTICLE http://zookeys.pensoft.net Launched to accelerate biodiversity research Review of the subgenus Plumiger of Myrmecophyes, with description of a new species (Heteroptera, Miridae, Halticini) Fedor V. Konstantinov1,2, Nikolay Simov3 1 Department of Entomology, Faculty of Biology, St. Petersburg State University, Universitetskaya nab. 7/9, St. Petersburg 199034, Russia 2 Zoological Institute, Russian Academy of Sciences, Universitetskaya nab. 1, St. Petersburg 199034, Russia 3 National Museum of Natural History, Bulgarian Academy of Sciences, 1 Tsar Osvoboditel Blvd., 1000 Sofia, Bulgaria Corresponding author: Fedor V. Konstantinov ([email protected]) Academic editor: A. Wheeler | Received 26 October 2017 | Accepted 19 April 2018 | Published 15 November 2018 http://zoobank.org/CC96882F-7123-4A2D-8345-7C5E36F817C1 Citation: Konstantinov FV, Simov N (2018) Review of the subgenus Plumiger of Myrmecophyes, with description of a new species (Heteroptera, Miridae, Halticini). In: Wheeler Jr AG (Ed.) A Festschrift Recognizing Thomas J. Henry for a Lifetime of Contributions to Heteropteran Systematics. ZooKeys 796: 215–239. https://doi.org/10.3897/zookeys.796.21877 Abstract The Caucasian subgenus Plumiger Horváth, 1927 of the halticine genus Myrmecophyes Fieber, 1870 is revised. A key, updated diagnoses, and data on distribution are given for the subgenus and its four species, including M. tomi sp. n. (Georgia and Dagestan), and the previously unknown male of M. armeniacus Drapolyuk, 1989. Illustrations of the male and female genitalia, photographs of the dorsal habitus, and SEM micrographs of selected structures are provided for all species of the subgenus. -

"Isometopinae" FIEB : "Miridae", "Heteroptera" and Their Intrarelationships
Title: Systematic position of "Isometopinae" FIEB : "Miridae", "Heteroptera" and their intrarelationships Author: Aleksander Herczek Citation style: Herczek Aleksander. (1993). Systematic position of "Isometopinae" FIEB : "Miridae", "Heteroptera" and their intrarelationships. Katowice : Uniwersytet Śląski Aleksander Herczek Systematic position of Isometopinae FIEB. (Miridae, Heteroptera) and their intrarelationships }/ f Uniwersytet Śląski • Katowice 1993 Systematic position of Isometopinae FIEB. (Miridae, Heteroptera) and their intrarelationships Prace Naukowe Uniwersytetu Śląskiego w Katowicach nr 1357 Aleksander Herczek ■ *1 Systematic position of Isometopinae FIEB. (Miridae, Heteroptera) and their intrarelationships Uniwersytet Śląski Katowice 1993 Editor of the Series: Biology LESŁAW BADURA Reviewers WOJCIECH GOSZCZYŃSKI, JAN KOTEJA Executive Editor GRAŻYNA WOJDAŁA Technical Editor ALICJA ZAJĄCZKOWSKA Proof-reader JERZY STENCEL Copyright © 1993 by Uniwersytet Śląski All rights reserved ISSN 0208-6336 ISBN 83-226-0515-3 Published by Uniwersytet Śląski ul. Bankowa 12B, 40-007 Katowice First impression. Edition: 220+ 50 copies. Printed sheets: 5,5. Publishing sheets: 7,5. Passed to the Printing Works in June, 1993. Signed for printing and printing finished in September, 1993. Order No. 326/93 Price: zl 25 000,— Printed by Drukarnia Uniwersytetu Śląskiego ul. 3 Maja 12, 40-096 Katowice Contents 1. Introduction.......................................................................................................... 7 2. Historical outline -

Paride Dioli Gli Eterotteri (Heteroptera) Del Monte Barro
Paride Dioli Gli Eterotteri (Heteroptera) del Monte Barro I (Italia, Lombardia, Lecco) ~f, Riassunto - Nel corso di una ricerca sugli Eterotteri del Monte Barro sono state censite 169 specie, di cui 13 vengono segnalate per la prima volta in Lombardia. Esse sono: Bothynotus pilosus, Dicyphus annulatus, Phytocoris dimidiatus, Pina• litus atomarius, Heterocordylus tumidicornis, Globiceps horvathi, Driophylocoris flavoquadrimaculatus, Harpocera thoraci• ca, Heterocapillus tigripes, Berytinus minor, Berytinus clavipes, Heterogaster cathariae e Megalonotus dilatatus. Sono state inoltre confrontate mediante una cluster analysis le nove principali stazioni di campionamento delle specie. Dal punto di vi• sta zoogeografico è emerso che la maggior parte delle specie presenta ampia distribuzione in Asia ed Europa, mentre l'e• lemento mediterraneo è scarsamente rappresentato, anche in relazione all'assenza di piante ospiti stenomediterranee. Abstract - Bugs (Heteroptera) from Monte Barro (Italy, Lombardy, Lecco). As a result of a research on the heteropteran fauna (Insecta, Heteroptera) of the Monte Barro (Lombardia, Italy) 169 species have been recorded: thirteen of them (Bothynotus pilosus, Dicyphus annulatus, Phytocoris dimidiatus, Pinalitus ato• marius, Heterocordylus tumidicornis, Globiceps horvathi, Driophylocoris flavoquadrimaculatus, Harpocera thoracica, Hete• rocapillus tigripes, Berytinus minor, Berytinus clavipes, Heterogaster cathariae and Megalonotus dilatatus) are new for Lom• bardia. The main sampling sites (sites 1-9) ha ve been compared -

An Annotated Catalog of the Iranian Miridae (Hemiptera: Heteroptera: Cimicomorpha)
Zootaxa 3845 (1): 001–101 ISSN 1175-5326 (print edition) www.mapress.com/zootaxa/ Monograph ZOOTAXA Copyright © 2014 Magnolia Press ISSN 1175-5334 (online edition) http://dx.doi.org/10.11646/zootaxa.3845.1.1 http://zoobank.org/urn:lsid:zoobank.org:pub:C77D93A3-6AB3-4887-8BBB-ADC9C584FFEC ZOOTAXA 3845 An annotated catalog of the Iranian Miridae (Hemiptera: Heteroptera: Cimicomorpha) HASSAN GHAHARI1 & FRÉDÉRIC CHÉROT2 1Department of Plant Protection, Shahre Rey Branch, Islamic Azad University, Tehran, Iran. E-mail: [email protected] 2DEMNA, DGO3, Service Public de Wallonie, Gembloux, Belgium, U. E. E-mail: [email protected] Magnolia Press Auckland, New Zealand Accepted by M. Malipatil: 15 May 2014; published: 30 Jul. 2014 HASSAN GHAHARI & FRÉDÉRIC CHÉROT An annotated catalog of the Iranian Miridae (Hemiptera: Heteroptera: Cimicomorpha) (Zootaxa 3845) 101 pp.; 30 cm. 30 Jul. 2014 ISBN 978-1-77557-463-7 (paperback) ISBN 978-1-77557-464-4 (Online edition) FIRST PUBLISHED IN 2014 BY Magnolia Press P.O. Box 41-383 Auckland 1346 New Zealand e-mail: [email protected] http://www.mapress.com/zootaxa/ © 2014 Magnolia Press All rights reserved. No part of this publication may be reproduced, stored, transmitted or disseminated, in any form, or by any means, without prior written permission from the publisher, to whom all requests to reproduce copyright material should be directed in writing. This authorization does not extend to any other kind of copying, by any means, in any form, and for any purpose other than private research use. ISSN 1175-5326 (Print edition) ISSN 1175-5334 (Online edition) 2 · Zootaxa 3845 (1) © 2014 Magnolia Press GHAHARI & CHÉROT Table of contents Abstract . -

Autumn 2011 Newsletter of the UK Heteroptera Recording Schemes 2Nd Series
Issue 17/18 v.1.1 Het News Autumn 2011 Newsletter of the UK Heteroptera Recording Schemes 2nd Series Circulation: An informal email newsletter circulated periodically to those interested in Heteroptera. Copyright: Text & drawings © 2011 Authors Photographs © 2011 Photographers Citation: Het News, 2nd Series, no.17/18, Spring/Autumn 2011 Editors: Our apologies for the belated publication of this year's issues, we hope that the record 30 pages in this combined issue are some compensation! Sheila Brooke: 18 Park Hill Toddington Dunstable Beds LU5 6AW — [email protected] Bernard Nau: 15 Park Hill Toddington Dunstable Beds LU5 6AW — [email protected] CONTENTS NOTICES: SOME LITERATURE ABSTRACTS ........................................... 16 Lookout for the Pondweed leafhopper ............................................................. 6 SPECIES NOTES. ................................................................18-20 Watch out for Oxycarenus lavaterae IN BRITAIN ...........................................15 Ranatra linearis, Corixa affinis, Notonecta glauca, Macrolophus spp., Contributions for next issue .................................................................................15 Conostethus venustus, Aphanus rolandri, Reduvius personatus, First incursion into Britain of Aloea australis ..................................................17 Elasmucha ferrugata Events for heteropterists .......................................................................................20 AROUND THE BRITISH ISLES............................................21-22 -

The Flora and Fauna of the Northwich Woodlands
The Flora and Fauna of the Northwich Woodlands Compiled by Paul M Hill Last updated: 23 rd August 2010 CONTENTS Plants 4 Mosses 8 Fungi 9 Bryophytes 10 Lichens 10 Beetles 11 Bees, Ants and Wasps 13 Sawflies 13 Parasitic / Gall Waps 14 True Bugs 14 Planthoppers and Aphids 14 Mayflies 14 Scorpianflies 15 Lacewings 15 Stoneflies 15 Caddisflies 15 Flies 15 Micro-moths 20 Butterflies 24 Macro-moths 24 Dragonflies and Damselflies 27 Earwigs 27 Grasshopper, Crickets and Groundhoppers 28 Amphipods 28 Wood and Water Louse 28 Spiders 28 Mites 29 Centipedes and Millipedes 29 Leeches 29 Snails and Slugs 30 Birds 31 Mammals 33 Amphibians and Reptiles 33 BOTANICAL Plants Equisetum arvense............. Field Horsetail Phleum bertolonii................ Smaller Cat's-tail Equisetum palustre............. Marsh Horsetail Phleum pratense ................ Timothy Equisetum sylvaticum......... Wood Horsetail Phragmites australis ........... Common Reed Larix decidua ..................... European Larch Poa annua.......................... Annual Meadow-grass Pinus nigra......................... Austrian Pine / Corsican Poa compressa .................. Flattened Meadow-grass Pine Poa pratensis ..................... Smooth Meadow-grass Pinus sylvestris .................. Scots Pine Poa humilis......................... Spreading Meadow-grass Taxus baccata.................... Yew Poa trivialis......................... Rough Meadow-grass Taxodium distichum ........... Swamp Cypress Puccinellia distans.............. Reflexed Saltmarsh-grass Ophioglossum vulgatum.... -

COI Barcoding of Plant Bugs (Insecta: Hemiptera: Miridae)
COI barcoding of plant bugs (Insecta: Hemiptera: Miridae) Junggon Kim and Sunghoon Jung Laboratory of Systematic Entomology, Department of Applied Biology, College of Agriculture and Life Sciences, Chungnam National University, Daejeon, Korea ABSTRACT The family Miridae is the most diverse and one of the most economically important groups in Heteroptera. However, identification of mirid species on the basis of morphology is difficult and time-consuming. In the present study, we evaluated the effectiveness of COI barcoding for 123 species of plant bugs in seven subfamilies. With the exception of three Apolygus species—A. lucorum, A. spinolae, and A. watajii (sub- family Mirinae)—each of the investigated species possessed a unique COI sequence. The average minimum interspecific genetic distance of congeners was approximately 37 times higher than the average maximum intraspecific genetic distance, indicating a significant barcoding gap. Despite having distinct morphological characters, A. lu- corum, A. spinolae, and A. watajii mixed and clustered together, suggesting taxonomic revision. Our findings indicate that COI barcoding represents a valuable identification tool for Miridae and can be economically viable in a variety of scientific research fields. Subjects Agricultural Science, Bioinformatics, Entomology, Molecular Biology, Taxonomy Keywords DNA barcoding, COI, Insects, Plant bugs, Miridae INTRODUCTION Heteroptera (Insecta: Hemiptera)—commonly termed true bugs—comprises the largest global group of hemimetabolous insects, having more -
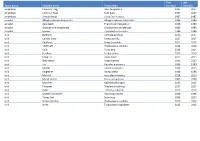
Taxon Group Common Name Taxon Name First Recorded Last
First Last Taxon group Common name Taxon name recorded recorded amphibian Common Frog Rana temporaria 1987 2017 amphibian Common Toad Bufo bufo 1987 2017 amphibian Smooth Newt Lissotriton vulgaris 1987 1987 annelid Alboglossiphonia heteroclita Alboglossiphonia heteroclita 1986 1986 annelid duck leech Theromyzon tessulatum 1986 1986 annelid Glossiphonia complanata Glossiphonia complanata 1986 1986 annelid leeches Erpobdella octoculata 1986 1986 bird Bullfinch Pyrrhula pyrrhula 2016 2017 bird Carrion Crow Corvus corone 2017 2017 bird Chaffinch Fringilla coelebs 2015 2017 bird Chiffchaff Phylloscopus collybita 2014 2016 bird Coot Fulica atra 2014 2014 bird Fieldfare Turdus pilaris 2015 2015 bird Great Tit Parus major 2015 2015 bird Grey Heron Ardea cinerea 2013 2017 bird Jay Garrulus glandarius 1999 1999 bird Kestrel Falco tinnunculus 1999 2015 bird Kingfisher Alcedo atthis 1986 1986 bird Mallard Anas platyrhynchos 2014 2015 bird Marsh Harrier Circus aeruginosus 2000 2000 bird Moorhen Gallinula chloropus 2015 2015 bird Pheasant Phasianus colchicus 2017 2017 bird Robin Erithacus rubecula 2017 2017 bird Spotted Flycatcher Muscicapa striata 1986 1986 bird Tawny Owl Strix aluco 2006 2015 bird Willow Warbler Phylloscopus trochilus 2015 2015 bird Wren Troglodytes troglodytes 2015 2015 bird Yellowhammer Emberiza citrinella 2000 2000 conifer Douglas Fir Pseudotsuga menziesii 2004 2004 conifer European Larch Larix decidua 2004 2004 conifer Lawson's Cypress Chamaecyparis lawsoniana 2004 2004 conifer Scots Pine Pinus sylvestris 1986 2004 crustacean -

Hemiptera of Israel
ANNALES ZOOLOGICI SOCIETATIS ZOOLOGICxE BOTANIME FENNICE 'VANAMO (ANN. ZOOL. SoC. 'VANAMO') ToM. 22. N:o 7. SUOMALAISEN ELA IN- JA KASVITIETEELLISE:N SEURAN VANAMON EULINTIETEELLISIX JULKAISUJA OSA 22. N:o 7. HEMIPTERA OF ISRAEL II R. LINNAVUORI HELSINKI 1961 Published by the Societas Zoologica Botanica Fennica )>Vanamo)) Address: Snellmaninkatu 9 - 11, Helsinki, Finland IHerausgeber: Societas Zoologica Botanica Fennica > Vanamo)> Anschrift: Snellmaninkatu 9 -11, Helsinki, FinnIand ANNALES ZOOLOGICI SOCIETATIS ZOOLOG1cAE BOTANICxE FENNICE 'VANAMO' (ANN. ZOOL. SOC. 'VANAMO') TOM. 22. N:o 7. SUOMALAISEN ELXIN- JA KASVITIETEELLISEN SEURAN VANAMON ELXINTIETEELLISIX JULKAISUJA OSA 22. N:o 7. HEMIPTERA OF ISRAEL 'II R. LINNAVUORI 22 Figures Selostus: Israelin nivelkarsaiset. II HELSINKI 1961 CONTENTS Page 1. Introduction ........................................ 1 2. Taxonomy and distribution of the species treated ................ ................ 1 Miridae (Continuation) .................. 1 Cimicidae ................. 35 Anthocoridae ................. - 35 Nabidae ......... 37 Reduzviidae ................................................... 38 Joppeicidae .......... 46 Aradidae ......... 46 Tingidae ......... 46 Piesmidae ......... 50 References ......... 50 Selostus .......... 51 Received 20. 1.1961 Printed 15. IX. 1961 Suomalaisen Kirjallisuuden Kirjapaino Oy Helsinki 19%1 1. INTRODUCTION This paper is a continuation of the author's previous survey (LINNAVUORI 1960) on the Hemiptera of Israel, based partly on the collections made by the author between June 12 and August 7, 1958, partly on revision of material from the con- siderable. collection at the University of Helsinki and several Israeli collections. As in the first part of this paper, all the material found by myself is marked I and that revised by me (!) in the present list. In other respects the reader is referred to the first part of this survey. 2. TAXONOMY AND DISTRIBUTION OF THE SPECIES TREATED Miridae (Continuation) Stenodema Lap. -

Miridae (Hemiptera: Heteroptera) Del Parque Natural De Aiako Harria (Gipuzkoa, País Vasco, Norte De La Península Ibérica)
Heteropterus Revista de Entomología 2005 Heteropterus Rev. Entomol. 5: 37-51 ISSN: 1579-0681 Miridae (Hemiptera: Heteroptera) del Parque Natural de Aiako Harria (Gipuzkoa, País Vasco, norte de la Península Ibérica) S. PAGOLA-CARTE1, I. ZABALEGUI2, J. RIBES3 1Azpeitia 3, 7. D; E-20010 Donostia (Gipuzkoa); E-mail: [email protected] 2Zikuñaga 44, 4º A; E-20120 Hernani (Gipuzkoa); E-mail: [email protected] 3València 123-125, ent., 3a; E-08011 Barcelona; E-mail: [email protected] Resumen Se enumeran y comentan las especies de míridos (Hemiptera: Heteroptera: Miridae) capturadas en el Parque Natural de Aiako Harria (Gipuzkoa, País Vasco). De un total de 71 taxones, 49 se citan por primera vez para la Comunidad Autónoma Vasca, constituyendo uno de ellos, Psallus (Hylopsallus) wagneri Ossiannilsson, 1953, el primer registro ibérico. Se establece la sinonimia Phylus (Phylus) melanocephalus (Linnaeus, 1767) = Phylus (Phylus) palliceps Fieber, 1861 syn. n. Palabras clave: Nueva sinonimia, Phylus (Phylus) melanocephalus (Linnaeus, 1767) = Phylus (Phylus) palliceps Fieber, 1861 syn. n., Miridae, Heteroptera, Parque Natural de Aiako Harria, País Vasco,Península Ibérica, nuevas citas. Laburpena Miridae (Hemiptera: Heteroptera) Aiako Harria Parke Naturalean (Gipuzkoa, Euskal Herria, Iberiar Penintsularen iparraldea) Aiako Harria Parke Naturalean (Gipuzkoa, Euskal Herria) harrapatutako mirido-espezieak (Hemiptera: Hete- roptera: Miridae) zerrendatu eta komentatzen dira. Aurkituriko 71 taxonetatik, 49 lehenengo aldiz aipatzen dira Euskal Autonomia Erkidegoan, eta bat, Psallus (Hylopsallus) wagneri Ossiannilsson, 1953, lehendabiziko aipua da Iberiar Penintsularako. Ondorengo sinonimia eratu da: Phylus (Phylus) melanocephalus (Linnaeus, 1767) = Phylus (Phylus) palliceps Fieber, 1861 syn. n. Gako-hitzak: Sinonimia berria, Phylus (Phylus) melanocephalus (Linnaeus, 1767) = Phylus (Phylus) palliceps Fieber, 1861 syn. -

Insecta, Heteroptera, Familia Miridae
Contribución al estudio de los hemípteros (Insecta, Heteroptera, Familia Miridae). Goula Goula, Marta ADVERTIMENT. La consulta d’aquesta tesi queda condicionada a l’acceptació de les següents condicions d'ús: La difusió d’aquesta tesi per mitjà del servei TDX (www.tesisenxarxa.net) ha estat autoritzada pels titulars dels drets de propietat intel·lectual únicament per a usos privats emmarcats en activitats d’investigació i docència. No s’autoritza la seva reproducció amb finalitats de lucre ni la seva difusió i posada a disposició des d’un lloc aliè al servei TDX. No s’autoritza la presentació del seu contingut en una finestra o marc aliè a TDX (framing). Aquesta reserva de drets afecta tant al resum de presentació de la tesi com als seus continguts. En la utilització o cita de parts de la tesi és obligat indicar el nom de la persona autora. ADVERTENCIA. La consulta de esta tesis queda condicionada a la aceptación de las siguientes condiciones de uso: La difusión de esta tesis por medio del servicio TDR (www.tesisenred.net) ha sido autorizada por los titulares de los derechos de propiedad intelectual únicamente para usos privados enmarcados en actividades de investigación y docencia. No se autoriza su reproducción con finalidades de lucro ni su difusión y puesta a disposición desde un sitio ajeno al servicio TDR. No se autoriza la presentación de su contenido en una ventana o marco ajeno a TDR (framing). Esta reserva de derechos afecta tanto al resumen de presentación de la tesis como a sus contenidos. En la utilización o cita de partes de la tesis es obligado indicar el nombre de la persona autora. -

NOTES ÉCOLOGIQUES SUR LES MIRIDAE (INSECTA-HETEROPTERA) OBSERVÉS EN BRETAGNE SUR LE CHÊNE Bernard Ehanno
NOTES ÉCOLOGIQUES SUR LES MIRIDAE (INSECTA-HETEROPTERA) OBSERVÉS EN BRETAGNE SUR LE CHÊNE Bernard Ehanno To cite this version: Bernard Ehanno. NOTES ÉCOLOGIQUES SUR LES MIRIDAE (INSECTA-HETEROPTERA) OB- SERVÉS EN BRETAGNE SUR LE CHÊNE. Vie et Milieu , Observatoire Océanologique - Laboratoire Arago, 1965, pp.517-534. hal-02940228 HAL Id: hal-02940228 https://hal.sorbonne-universite.fr/hal-02940228 Submitted on 16 Sep 2020 HAL is a multi-disciplinary open access L’archive ouverte pluridisciplinaire HAL, est archive for the deposit and dissemination of sci- destinée au dépôt et à la diffusion de documents entific research documents, whether they are pub- scientifiques de niveau recherche, publiés ou non, lished or not. The documents may come from émanant des établissements d’enseignement et de teaching and research institutions in France or recherche français ou étrangers, des laboratoires abroad, or from public or private research centers. publics ou privés. NOTES ÉCOLOGIQUES SUR LES MIRIDAE (INSECTA-HETEROPTERA) OBSERVÉS EN BRETAGNE SUR LE CHÊNE par Bernard EHANNO SOMMAIRE Certains Miridae se recontrent régulièrement sur les Chênes de Bre- tagne, d'autres ne sont capturés que fortuitement. Leur succession dans le temps est étudiée, particulièrement pour les espèces qui semblent effectuer leur cycle sur le Chêne. Les Insectes Hétéroptères Miridae ont donné lieu, ces dernières années, à de nombreux travaux se rapportant à leur systématique, à leur répartition, à leur biologie générale, notamment ceux de CHINA, DAVIS, KULLENBERG, LESTON, SIENKIEWICKZ, SLATER, SOUTH- WOOD, STEHLIK, STICHEL, WAGNER. Ces Insectes n'avaient pas été recherchés de manière systéma- tique en Bretagne bien que les autres Hétéroptères terrestres de cette région aient fait l'objet, en particulier, des nombreuses publi- cations de J.