I-Mak) in Support of Respondents
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

The Pulitzer Prizes 2020 Winne
WINNERS AND FINALISTS 1917 TO PRESENT TABLE OF CONTENTS Excerpts from the Plan of Award ..............................................................2 PULITZER PRIZES IN JOURNALISM Public Service ...........................................................................................6 Reporting ...............................................................................................24 Local Reporting .....................................................................................27 Local Reporting, Edition Time ..............................................................32 Local General or Spot News Reporting ..................................................33 General News Reporting ........................................................................36 Spot News Reporting ............................................................................38 Breaking News Reporting .....................................................................39 Local Reporting, No Edition Time .......................................................45 Local Investigative or Specialized Reporting .........................................47 Investigative Reporting ..........................................................................50 Explanatory Journalism .........................................................................61 Explanatory Reporting ...........................................................................64 Specialized Reporting .............................................................................70 -

Playing with Safety: Dangerous Toys and the Role of America's Civil
Playing with Safety: Dangerous Toys and the Role of America’s Civil Justice System December 2010 Playing with Safety: Dangerous Toys and the Role of America’s Civil Justice System 1 Table of Contents Introduction 3 Danger in Familiar Places 4 Lead 6 Toxic Substances 8 Magnets 10 Conclusion 13 Appendix: Resources for Consumers 14 Endnotes 15 Playing with Safety: Dangerous Toys and the Role of America’s Civil Justice System 2 Introduction Today’s toys are not your parents’ toys. Toys have grown in sophistication and technological advancement, but so have their dangers. In 1970, the most popular toy on the market was the then brand new Nerf Ball. Forty years later, the Nerf is still popular but has morphed into a “Blaster” – armed with a fl ip-up sight, red dot light beam, and shoulder stock with an extra ammo clip – and had to be recalled after the gun’s mechanism injured more than 45 children.1 While most parents have always had the common sense to watch for small objects that might choke a child or sharp pieces that might cause harm, today’s toys feature unseen hazards. Now, the danger comes from lead, cadmium, asbestos, and other carcinogens undetectable to the eye, or small, innocent-looking magnets that can rip a child apart from the inside. Since 1974, the Consumer Product Safety Commission (CPSC) has issued more than 850 recalls for toy products. In 2007, 45 million toys had to be recalled.2 Between 2004 and 2008, toy-related injuries increased 12 percent, and over the last 10 years, toy-related injuries have increased 54 percent.3 This increase in the number of injuries to children every year has coincided with a marked increase in imported toys. -

2006-07 Annual Report
����������������������������� the chicago council on global affairs 1 The Chicago Council on Global Affairs, founded in 1922 as The Chicago Council on Foreign Relations, is a leading independent, nonpartisan organization committed to influencing the discourse on global issues through contributions to opinion and policy formation, leadership dialogue, and public learning. The Chicago Council brings the world to Chicago by hosting public programs and private events featuring world leaders and experts with diverse views on a wide range of global topics. Through task forces, conferences, studies, and leadership dialogue, the Council brings Chicago’s ideas and opinions to the world. 2 the chicago council on global affairs table of contents the chicago council on global affairs 3 Message from the Chairman The world has undergone On September 1, 2006, The Chicago Council on tremendous change since Foreign Relations became The Chicago Council on The Chicago Council was Global Affairs. The new name respects the Council’s founded in 1922, when heritage – a commitment to nonpartisanship and public nation-states dominated education – while it signals an understanding of the the international stage. changing world and reflects the Council’s increased Balance of power, national efforts to contribute to national and international security, statecraft, and discussions in a global era. diplomacy were foremost Changes at The Chicago Council are evident on on the agenda. many fronts – more and new programs, larger and more Lester Crown Today, our world diverse audiences, a step-up in the pace of task force is shaped increasingly by forces far beyond national reports and conferences, heightened visibility, increased capitals. -
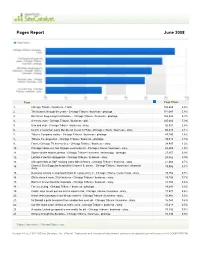
Pages Report June 2008
Pages Report June 2008 Page Page Views 1. Chicago Tribune / business - Front. 189,465 6.4% 2. Tim Russert through the years - Chicago Tribune / business - photoga. 171,047 5.8% 3. Bill Gates: Beginnings to billionaire - Chicago Tribune / business - photoga. 155,826 5.3% 4. Anchors, man - Chicago Tribune / business - poll. 145,666 5.0% 5. Uno and only - Chicago Tribune / business - story. 82,501 2.8% 6. Ex-Ch. 2 newsman Larry Mendte off the air in Phila - Chicago Tribune / business - story. 60,610 2.1% 7. Tribune Company history - Chicago Tribune / business - photoga. 47,740 1.6% 8. Tribune Co. properties - Chicago Tribune / business - photoga. 39,013 1.3% 9. Former Chicago TV anchor dies - Chicago Tribune / business - story. 34,497 1.2% 10. Chicago makes cut, has Olympic-size to-do list - Chicago Tribune / business - story. 28,659 1.0% 11. Space shuttle Atlantis photos - Chicago Tribune / business / technology - photoga. 27,457 0.9% 12. LaSalle s law firm disappears - Chicago Tribune / business - story. 25,362 0.9% 13. Chicago holds as S&P housing index falls at fastes - Chicago Tribune / business - story. 21,462 0.7% 14. Channel 32 s Suppelsa headed for Channel 9, source - Chicago Tribune / business / columnist - 19,986 0.7% story. 15. Business owners in swamped Chain O Lakes worry fl - Chicago Tribune / news / local - story. 19,752 0.7% 16. GM to close 4 truck, SUV factories - Chicago Tribune / business - story. 19,705 0.7% 17. Ball Girl scores buzz for Gatorade - Chicago Tribune / business - story. 18,760 0.6% 18. -

CHICAGO TRIBUNE Media Group RECE!V'.=D
CHICAGO TRIBUNE media group RECE!V'.=D !Nll~:::,'r.1.c: 11111 i·~·y Sold To: REGUL.r:.70R:1· 2oi~lv11ssioN Indiana Utility Regulatory Commission - CU00411916 101 W Washington St Ste 1500 lndianapolis,IN 46204-3419 Bill To: Indiana Utility Regulatory Commission - CU00411916 101 W Washington St Ste 1500 lndianapolis,IN 46204-3419 Proof of Publication Order Number: 6014531 Purchase Order: CAUSE NO. 44403 TOSIC 9 & 4 State of Indiana ) ) ss: Jasper, La Porte, Lake, Newton, Porter, & Starke County I, Stefanie Sobie , a principal clerk of Post Tribune newspaper of general circulation printed and published in the English language in the city of Crown Point in state and county afore-said, and that the printed matter attached hereto is a true copy, which was duly published in said paper for 1 time(s), the date(s) of publication being as follows: Dec 01. 2018. The undersigned further states that the Post Tribune newspaper(s) maintains an Internet website, which is located at http://classifieds.chicagotribune.com/classifieds?category=public_notice website and that a copy of the above referenced printed · atter was posted on such website on the date(s) of publication set forth above. 435 N. Michigan Ave. Chicago, IL Chicago Tribune - chicagotribune.com 160 N Stetson A venue, Chicago, IL 60601 (312) 222-2222 - Fax: (312) 222-4014 CHICAGO TRIBUNE media group LEGAL NOTICE OF EVI· DENTIARY HEARING INDIANA UTILITY REGU· LATORY COMMISSION CAUSE NO. 44403 T[).. SIC 9 AND CAUSE NO. 44403 TOSIC 4 VERIFlED PETITION OF NORTHERN INDIANA PUB LIC SERVICE COMPANY LLC FOR (1) APPROVAL OF AN ADJUSTMENT TO ITS GAS SERVICE RATES THROUGH ITS TRANS MISSION, DISTRIBUTION, AND STORAGE SVSTEM IMPROVEMENT CHARGE ("TDSIC'1 RAlE SCHED ULE; (2) AUTHORITY TO DEFER 20% OF THE AP PROVED CAPITAL EX PENDITURES AND TOSIC COSTS FOR RECOVERY IN PETITIONER'S NEXT GENERAL RATE CASE; (3) APPROVAL OF PETITION ER'S UPDATED 71ii\"EAR GAS PLAN, INCLUDING ACTUAL AND PROPOSED ESTIMATED CAPITAL EX PENDITURES AND TOSIC COSTS THAT EXCEED THE APPROVED AMOUNTS JN CAUSE NO. -

White House News Photographers’ Association
WHITE HOUSE NEWS PHOTOGRAPHERS’ ASSOCIATION PO 7119, Washington, DC 20044–7119 www.whnpa.org OFFICERS Susan A. Walsh, Associated Press, President John Poole, WashingtonPost.com, Vice President Nikki Kahn, Washington Post, Secretary Jonathan Elswick, Associated Press, Treasurer EXECUTIVE BOARD Susan Biddle (Washington Post) Dennis Brack (Black Star) Cliff Owen (AOL) Ed Eaves (NBC News) Stu Cohen (Freelance) Pierre Kattar, (Washingtonpost.com) Pete Souza, Contest Chair (Chicago Tribune) Contest Chair, Television (Open) Leighton Mark, Education Chair (Associated Press) MEMBERS REPRESENTED Abraham, Mark: Freelance Brisson, Stephane: CTV Television Adlerblum, Robin: CBS News Brooks, Dudley: Washington Post Alberter Jr., William: CNN Brown, Stephen: Allen, Tom: Bruce Woodall, Andrea: Washington Post Alleruzzo, Maya: Washington Times Bryan, Beverly: WJLA–TV Apt Johnson, Roslyn: Freelance Bui, Khue: Freelance Archambault, Charles: US News & World Report Burgess, Robert Harrison: Freelance Arias, Juana: Washington Post Burke, Lauren Victoria: Freelance Arrington, Clyde Steven: ABC News Burnett, David: Contact Press Images Attlee, Tracey: Freelance Butler, Francis: Freelance Auth, William: US News & World Report Cain, Stephen: ABC–TV Baker, David: ITN Calvert, Mary F.: Washington Times Ballard, Karen: Freelance Cameron, Gary: Reuters Baughman, J. Ross: Washington Times Carioti, Ricky: Washington Post Baylen, Elizabeth Olivia: Washington Times Casey, Sean: NBC4 Beiser, H. Darr: USA Today Cassetta, Guido: Freelance Biddle, Susan: Washington Post -

JEA/NSPA Fall National High School Journalism Convention November 1-4, 2018 • Hyatt Regency Chicago
JEA/NSPA Fall National High School Journalism Convention November 1-4, 2018 • Hyatt Regency Chicago JEA/NSPA Fall 2018 • CHICAGO — 1 PARK SCHOLAR PROGRAM A once-in-a-lifetime opportunity awaits outstanding high school seniors. A full scholarship for at least 10 exceptional communications students that covers the four-year cost of attendance at Ithaca College. Take a chance. Seize an opportunity. Change your life. Study at one of the most prestigious communications schools in the country—Ithaca College’s Roy H. Park School of Communications. Join a group of bright, competitive, and energetic students who are committed to using mass communication to make a positive impact on the world. To apply for this remarkable opportunity and to learn more, contact the Park Scholar Program director at [email protected] or 607-274-3089. ithaca.edu/parkscholars 2 — JEA/NSPA Fall 2018 • CHICAGO Twitter: @nhsjc/#nhsjc PARK SCHOLAR CONTENTS 4 Convention Officials PROGRAM 5 Local Team/One Story A once-in-a-lifetime opportunity awaits 6 Convention Rules/App outstanding high school seniors. 7 Keynote Speaker A full scholarship for at least 10 exceptional communications 8 Special Activities students that covers the four-year cost of attendance at Ithaca College. 10 Featured Speakers 14 Exhibitors/Advertisers 15 Sponsors 18 JEA Awards 20 NSPA Awards 25 Thursday at a Glance 25 Thursday Sessions 32 Friday at a Glance 39 Write-off Rooms 40 Friday Sessions 68 Saturday at a Glance 75 Saturday Sessions Take a chance. 98 Speaker Bios Seize an opportunity. 130 Hotel Floor Plans Change your life. Study at one of the most prestigious communications schools in the country—Ithaca College’s Roy H. -

Silurian News March 2014
Society of the Silurians EXCELLENCE IN JOURNALISM AWARDS DINNER The Players Club 16 Gramercy Park South Thursday, May 15, 2014 Drinks: 6 p.m. Dinner: 7:15 p.m. Meet Old Friends and Award Winners Published by The Society of The Silurians, Inc., an organization (212) 532-0887 of veteran New York City journalists founded in 1924 Please Save the Date. Reservation forms will be mailed soon. MARCH 2014 From Print to Digital: My Turbulent Path BY STEPHEN B. SHEPARD FROM TO sometimes with great reluctance, I came hen I first considered taking to see the value of the new technologies. on the role of founding dean I slowly realized that digital technology Wof a brand-new journalism would enrich journalism, creating an in- school, I initially thought of it as a per- teractive, multimedia form of storytelling sonal capstone, the culmination of a life- that invited community participation, that time in journalism. Having been a senior could be personalized, that could be de- editor at Newsweek, editor of the Satur- livered on a vast array of mobile devices, day Review, and editor-in-chief of that could be consumed globally, that BusinessWeek for more than 20 years, I could be distributed using social media. saw my new posting as a chance to pass And so, I finally managed to embrace the on my experience to the next generation. changes necessary to create a new Boy, was I wrong. As the journalism world school for a new age. Stephen B. Shepard became the founding dean of the Graduate School of changed in content and delivery, I was My personal passage is, of course, a Journalism at the City University of New York in March 2005. -

Charles Forelle, James Bandler and Mark Maremont of the Wall Street Journal Win Goldsmith Prize for Investigative Reporting
CONTACT: Molly Lanzarotta March 5, 2013 Harvard Kennedy School Communications 617-495-1144 Patricia Callahan, Sam Roe and Michael Hawthorne of the Chicago Tribune Win Goldsmith Prize for Investigative Reporting CAMBRIDGE, MASS – The $25,000 Goldsmith Prize for Investigative Reporting has been awarded to Patricia Callahan, Sam Roe and Michael Hawthorne of the Chicago Tribune by the Joan Shorenstein Center on the Press, Politics and Public Policy for their investigative report “Playing with Fire." The Shorenstein Center is part of the John F. Kennedy School of Government at Harvard University. The Chicago Tribune’s investigative series revealed how a deceptive campaign by the chemical and tobacco industries brought toxic flame retardants into people’s homes and bodies, despite the fact that the dangerous chemicals don’t work as promised. As a result of the investigation, the U.S. Senate revived toxic chemical reform legislation and California moved to revamp the rules responsible for the presence of dangerous chemicals in furniture sold nationwide. “The judges this year were especially struck by the initiative shown in recognizing a very important policy issue embedded in something as familiar and unthreatening as a sofa,” said Alex S. Jones, Director of the Shorenstein Center. “It goes to prove the importance of not just looking, but seeing and acting.” Launched in 1991, the Goldsmith Prize for Investigative Reporting honors journalism which promotes more effective and ethical conduct of government, the making of public policy, or the practice of politics by disclosing excessive secrecy, impropriety and mismanagement. The five finalists for the Goldsmith Prize for Investigative Reporting were: Alan Judd, Heather Vogell, John Perry, M.B. -

Press Galleries* Rules Governing Press Galleries
PRESS GALLERIES* SENATE PRESS GALLERY The Capitol, Room S–316, phone 224–0241 Director.—Robert E. Petersen, Jr. Deputy Director.—S. Joseph Keenan Media Coordinators: Merri I. Baker Wendy A. Oscarson James D. Saris Amy Harkins HOUSE PRESS GALLERY The Capitol, Room H–315, phone 225–3945, 225–6722 Superintendent.—Jerry L. Gallegos Deputy Superintendent.—Justin J. Supon Assistant Superintendents: Emily T. Dupree Ric Andersen Cris M. King Lori Michelle Hodo STANDING COMMITTEE OF CORRESPONDENTS Curt Anderson, The Associated Press, Chairman Jake Thompson, Omaha World-Herald, Secretary James Kuhnhenn, Knight Rider William Roberts, Bloomberg News Donna M. Smith, Reuters RULES GOVERNING PRESS GALLERIES 1. Administration of the press galleries shall be vested in a Standing Committee of Cor- respondents elected by accredited members of the galleries. The Committee shall consist of five persons elected to serve for terms of two years. Provided, however, that at the election in January 1951, the three candidates receiving the highest number of votes shall serve for two years and the remaining two for one year. Thereafter, three members shall be elected in odd-numbered years and two in even-numbered years. Elections shall be held in January. The Committee shall elect its own chairman and secretary. Vacancies on the Committee shall be filled by special election to be called by the Standing Committee. 2. Persons desiring admission to the press galleries of Congress shall make application in accordance with Rule 34 of the House of Representatives, subject to the direction and control of the Speaker and Rule 33 of the Senate, which rules shall be interpreted and administered by the Standing Committee of Correspondents, subject to the review and an approval by the Senate Committee on Rules and Administration. -

Juvenile Confessions and Exculpatory DNA in Cook County, IL Joshua A
Northwestern University School of Law Northwestern University School of Law Scholarly Commons Faculty Working Papers 2012 Convenient Scapegoats: Juvenile Confessions and Exculpatory DNA in Cook County, IL Joshua A. Tepfer Northwestern University School of Law, [email protected] Craig M. Cooley Cardozo Law School Tara Thompson University of Chicago Law School Repository Citation Tepfer, Joshua A.; Cooley, Craig M.; and Thompson, Tara, "Convenient Scapegoats: Juvenile Confessions and Exculpatory DNA in Cook County, IL" (2012). Faculty Working Papers. Paper 221. http://scholarlycommons.law.northwestern.edu/facultyworkingpapers/221 This Article is brought to you for free and open access by Northwestern University School of Law Scholarly Commons. It has been accepted for inclusion in Faculty Working Papers by an authorized administrator of Northwestern University School of Law Scholarly Commons. CONVENIENT SCAPEGOATS: JUVENILE CONFESSIONS AND EXCULPATORY DNA IN COOK COUNTY, IL Joshua A. Tepfer, Craig M. Cooley, & Tara Thompson In December 2001, the Chicago Tribune, led by reporters Ken Armstrong, Steve Mills, and Maurice Possley, published a series of investigative reports entitled ―Cops and Confessions.‖ Starting from 1991, these muckraking journalists waded through court documents and police reports of thousands of murder investigations in Cook County, Illinois.1 What they found was appalling: In at least 247 murder cases over this ten-year period, the police obtained incriminating statements that ―were thrown out by the courts -

Miami Herald, Nuevo Herald and Radio Martí Teaching Note CSJ-10-0026.3
CSJ‐ 10 ‐ 0026.3 When the story is us: Miami Herald, Nuevo Herald and Radio Martí Teaching Note Case Summary The press, as the journalist Walter Lippman famously wrote, “is like the beam of a searchlight that moves restlessly about, bringing one episode and then another out of darkness.” Typically that light focuses outwards, highlighting events and issues that lie beyond the world of journalism. But media attention can also be turned inwards, so that journalists themselves become the center of the story. This case focuses on one such occasion when journalists became the news. In September 2006, the Miami Herald (TMH) ran a front page story by reporter Oscar Corral that reported 10 local journalists had accepted money from a US government‐backed broadcast that opposed Cuba’s communist leader, Fidel Castro. Three of the journalists who had been paid by Radio/TV Martí worked at El Nuevo Herald (ENH), TMH’s Spanish‐language sister publication that was also owned by the Miami Herald Publishing Company. Publisher Jesús Díaz, Jr. fired the reporters on the eve of the story’s publication, a step that drew strong reaction from journalists at both papers, as well as the Cuban‐American community that accused the Miami Herald of racism. As feelings intensified, Miami Herald columnist Carl Hiassen submitted a piece that took a humorous approach to the story. Wary of exacerbating tensions, Díaz spiked the column. With Hiassen now threatening to quit, and ENH running a counter story to the Miami Herald’s that highlighted journalists who worked for other government‐funded stations with no consequences, matters moved up the chain of command to the McClatchy Company that owned both publications.