GALVANIZING HANDBOOK Foreword
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Wet Storage Stain
A Guide to Minimizing and Treating Wet Storage Stain on Hot-Dip Galvanized Steel Hot-Dip Galvanizing for Corrosion Protection Hot-dip galvanizing, used in myriad applications, combats corrosion throughout the world. Hot-dip galvanized steel is the most economical, maintenance-free corrosion protection system available. A key feature of hot-dip galvanized (HDG) products is longevity in various environments. The time to first maintenance of hot-dip galvanized steel is directly proportional to the thickness of the zinc coating; therefore, it is important to monitor any issue that may decrease the coating thickness. Like any manufacturing process, hot-dip galvanizing requires an inspection of the finished product to ensure compliance with applicable specifications. One factor closely scrutinized during inspection of hot-dip galvanized steel is the finish and appearance of the galvanized surface. Some aspects of the final appearance can be controlled by the galvanizer while others cannot. One surface condition is known as wet storage stain (commonly referred to as white rust), which can occur after galvanizing. San Diego Central Library utilizes galvanized steel © 2014 American Galvanizers Association. The material provided herein has been developed to provide accurate and authoritative information about after-fabrication hot-dip galvanized steel. This material provides general information only and is not intended as a substitute for competent professional examination and verification as to suitability and applicability. The information provided herein is not intended as a representation or warranty on the part of the AGA. Anyone making use of this information assumes all liability arising from such use. 2 American Galvanizers Association Figure 1: Wet Storage Stain What is We t Storage Stain? Wet storage stain (Figure 1) is a white or gray powdery Once the galvanized zinc coating is exposed to free deposit that can develop on newly galvanized articles. -

Guide to Preparing Hot-Dip Galvanized Steel for Paint
Guide to Preparing Hot-Dip Galvanized Steel for Paint Learn five key steps to prepare your next hot-dip galvanized steel project for paint. CONTENTS: Communicate with the Galvanizer . 4 Determine the Condition of the Surface . 5 • Newly Galvanized • Partially Weathered • Fully Weathered Clean the Surface . 6 • Remove bumps, runs, and drips • Remove organic materials • Rinse and dry Profile the Surface . 9 • Sweep Blasting • Wash Primer • Acrylic Pretreatment • Surface Grinding Painting . 10 Summary . 11 © 2011 American Galvanizers Association. The material provided herein has been developed to provide accurate and authoritative information about after-fabrication hot-dip galvanized steel. This material provides general information only and is not intended as a substitute for competent professional examination and verification as to suitability and applicability. The information provided herein is not intended as a representation or warranty on the part of the AGA. Anyone making use of this information assumes all liability arising from such use. ~ 2 ~ American Galvanizers Association Guide to Preparing Hot-Dip Galvanized Steel for Paint The Thurston Avenue Bridge utilizes a duplex system of paint over hot-dip galvanized (HDG) steel for corrosion protection Duplex systems, such as paint over hot-dip galvanized steel, have been used for decades as a means to enhance corrosion protection. Painting over hot-dip galvanized steel provides the owner/specifier with effective corrosion protection, while still allowing the structure to be painted for color or aesthetic reasons. Duplex systems are becoming increasingly common and popular because of this combination of benefits. Successfully painting over hot-dip galvanized steel is relatively simple. Just like painting on any other surface, proper surface preparation is key to creating an effective bond between the paint and the galvanized surface. -

The Engineers & Architects' Guide
THE ENGINEERS CI/SfB & ARCHITECTS' Yu1 GUIDE: HOT DIP GALVANIZING Galvanizers Association provides RIBA CPD assessed material: Literature The Engineers & Architects' Guide: Hot Dip Galvanizing The Engineers & Architects' Guide to Nuts and Bolts Seminar "Corrosion protection of steel by Hot Dip Galvanizing" For further information contact Nicky Smith on 0121 355 8838 or email [email protected] Front Cover: Bellmouth Passage Footbridge, Techniker & Patel Taylor, Peter Cook / View THE ENGINEERS & ARCHITECTS' GUIDE: HOT DIP GALVANIZING Galvanizers Association would like to know what you think of our services and the information we provide. Please use the response sheet on page 51 for your comments on the Guide or for any further information and advice that you may require. If you prefer, an online comment form is available at www.hdg.org.uk/feedback THE ENGINEERS & ARCHITECTS' GUIDE HOT DIP GALVANIZING INTRODUCTION STEEL IS A STRONG, VERSATILE AND INEXPENSIVE Hot dip galvanizing has many benefits Our technical team is on hand to give an MATERIAL WITH USES IN MANY DIFFERENT as a method of corrosion protection. immediate answer to all your queries about Importantly, it will: galvanizing. GA also monitors regulations, INDUSTRIES. IT HAS, HOWEVER, ONE MAJOR takes a leading role in standards development DISADVANTAGE: IT IS PRONE TO CORROSION, s¬PROVIDE¬STEEL¬WITH¬A¬COATING¬WHICH and oversees research projects to support EVEN IN INTERIOR ENVIRONMENTS CORROSION has a long, predictable and and develop the industry. PREVENTION IS THEREFORE ESSENTIAL IF STEEL maintenance free life. GA can also visit your company to give a STRUCTURES ARE TO BE ECONOMICAL. -
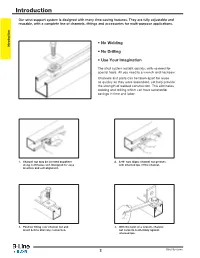
Introduction
Introduction Our strut support system is designed with many time-saving features. They are fully adjustable and reusable, with a complete line of channels, fittings and accessories for multi-purpose applications. • No Welding Introduction • No Drilling • Use Your Imagination The strut system installs quickly, with no need for special tools. All you need is a wrench and hacksaw. Channels and parts can be taken apart for reuse as quickly as they were assembled, yet help provide the strength of welded construction. This eliminates welding and drilling which can have substantial savings in time and labor. 1. Channel nut may be inserted anywhere 2. A 90° turn aligns channel nut grooves along continuous slot. Designed for easy with inturned lips of the channel. insertion and self-alignment. 3. Position fitting over channel nut and 4. With the twist of a wrench, channel insert bolt to start any connection. nut locks its teeth firmly against inturned lips. 2 Strut Systems Table of Contents Our strut system provides an economical solution for electrical, mechanical and industrial supports with an unlimited variety of applications in the construction industry. Introduction Electrical Applications • Lighting Fixture Supports • Raceway Systems • Trapeze Hangers • Pipe & Conduit Supports • Cable Tray Supports • Beam Adjustments Mechanical Applications • Piping Racks • Tunnel Pipe Stanchions • Concrete Inserts • Beam Attachments • Pipe Risers Industrial Applications • Racks and Shelving • Partitions • Production Line Supports • Trolley Systems • Wall Framing Strut Systems 3 Technical Data MATERIALS Carbon Steel Aluminum - Channels made from high-quality carbon Standard aluminum channel is extruded concentrated levels of Chlorine, [ Cl ]. steel are continuously roll formed to from aluminum alloy 6063-T6. -

Hot-Dip Galvanizing for Corrosion Prevention a Guide to Specifying and Inspecting Hot-Dip Galvanized Reinforcing Steel
Hot-Dip Galvanizing for Corrosion Prevention A Guide to Specifying and Inspecting Hot-Dip Galvanized Reinforcing Steel American Galvanizers Association 6881 South Holly Circle, Suite 108 Centennial, CO 80112 USA 1-800-HOT-SPEC www.galvanizeit.org Hot-Dip Galvanizing for Corrosion Protection: A Guide to Specifying and Inspecting Hot-Dip Galvanized Reinforcing Steel Table of Contents: Corrosion Protection for Steel Inspection Steel Corrosion . .3 How Zinc Prevents Steel from Corrosion . .4 Coating Thickness . .9 Barrier Protection . .4 Magnetic Thickness Measurements . .10 Cathodic Protection . .4 Stripping Method . .10 Weighing Bars . .10 Microscopy . .10 Design, Fabrication, and Installation Sampling Statistics . .10 Factors Affecting Coating Thickness . .10 Design . .5 Coating Appearance . .11 Steel Selection . .5 Detailing Reinforcement . .5 Visual Inspection Guide . .11 Dissimilar Metals in Concrete . .5 Bare Spots . .11 Dross Protrusions . .11 Fabrication . .6 Blisters and Slivers . .11 Bending Bars . .6 Flux Inclusions . .11 Storage and Handling . .6 Lumps and Runs . .11 Installation . .6 Zinc Skimmings . .12 Welding . .6 Gray or Mottled Coating Appearance . .12 Local Repair of Coating . .6 Brown Staining . .12 Removal of Forms . .6 Rust Staining . .12 Wet Storage Stain . .12 Galvanized Reinforcing Steel Testing . .12 The Hot-Dip Galvanizing Process Bond Strength Test . .12 Surface Preparation . .6 Chromate Finish Test . .12 Galvanizing . .7 Embrittlement Test . .12 Accelerated Aging Test . .12 Physical Properties of Hot-Dip Galvanized Coatings The Metallurgical Bond . .7 Field Performance of Galvanized Reinforcement Bermuda . .13 Impact and Abrasion Resistance . .8 Corner and Edge Protection . .8 New York State Thruway Authority . .13 Complete Coating . .8 Pennsylvania DOT . .14 Additional Resources . .15 Mechanical Properties of Galvanized Steel Ductility and Yield/Tensile Strength . -
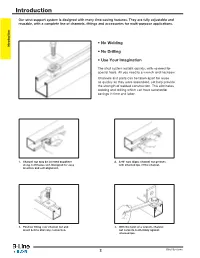
Introduction
Introduction Our strut support system is designed with many time-saving features. They are fully adjustable and reusable, with a complete line of channels, fittings and accessories for multi-purpose applications. • No Welding Introduction • No Drilling • Use Your Imagination The strut system installs quickly, with no need for special tools. All you need is a wrench and hacksaw. Channels and parts can be taken apart for reuse as quickly as they were assembled, yet help provide the strength of welded construction. This eliminates welding and drilling which can have substantial savings in time and labor. 1. Channel nut may be inserted anywhere 2. A 90° turn aligns channel nut grooves along continuous slot. Designed for easy with inturned lips of the channel. insertion and self-alignment. 3. Position fitting over channel nut and 4. With the twist of a wrench, channel insert bolt to start any connection. nut locks its teeth firmly against inturned lips. 2 Strut Systems Table of Contents Our strut system provides an economical solution for electrical, mechanical and industrial supports with an unlimited variety of applications in the construction industry. Introduction Electrical Applications • Lighting Fixture Supports • Raceway Systems • Trapeze Hangers • Pipe & Conduit Supports • Cable Tray Supports • Beam Adjustments Mechanical Applications • Piping Racks • Tunnel Pipe Stanchions • Concrete Inserts • Beam Attachments • Pipe Risers Industrial Applications • Racks and Shelving • Partitions • Production Line Supports • Trolley Systems • Wall Framing Strut Systems 3 Technical Data MATERIALS Carbon Steel Aluminum - Channels made from high-quality carbon Standard aluminum channel is extruded concentrated levels of Chlorine, [ Cl ]. steel are continuously roll formed to from aluminum alloy 6063-T6. -

Balmoral Tanks
BALMORAL TANKS CYLINDRICAL STEEL TANK STANDARD SPECIFICATION UK Designed and manufactured www.balmoraltanks.com CYLINDRICAL WATER TANKS | TECHNICAL SPECIFICATION Contents Balmoral Tanks 1 Tank overview 1 Balmoral Tanks specialises in the design and manufacture of GRP, steel sectional and 2 Tank design and key components 1 galvanized cylindrical steel bolted liquid storage 3 Standard tank accessories 4 tanks. These tanks are primarily used for the 4 Galvanizing 4 storage of water in the potable water, fire sprinkler and irrigation markets. 5 Epoxy and polyester coatings 5 6 Rubber tank liners 5 The galvanized cylindrical tanks are site 7 Liner repair 6 assembled using overlapping and bolted galvanized steel panels that are manufactured 8 Liner material specification: within the company’s facility in the UK. wras approved butyl 7 9 Liner material specification: edpm 7 Depending upon the application or design code, a choice of either a synthetic rubber membrane 10 Effect of chlorinated water on butyl liner or mastic is used to seal the tank together and epdm liners 8 with a plastic coated trough deck roof cover. 11 Hot dip galvanized sheet quality 8 These galvanized tanks provide an economical, 12 Renovating damaged coatings 10 reliable and low maintenance solution for water 13 Galvanized products: zinc patina 11 storage. 14 Wet storage stain: prevention and cure 12 Experience gained through the supply of over 15 Liner approval 13 5000 tanks worldwide and our ISO 9001:2008 accreditation ensures consistent quality of product and service for the design, manufacture and installation of liquid storage tanks. Scope This document has been written to provide relevant information for cylindrical galvanized water storage tanks designed and manufactured by Balmoral Tanks. -

TECHNICAL NOTE $5.00 on Cold-Formed Steel Construction
test $5.00 TECHNICAL NOTE $5.00 On Cold-Formed Steel Construction Cold-Formed Steel Engineers Institute ● Washington, DC ● www.cfsei.org ● 1-800-79-STEEL DURABILITY OF COLD-FORMED STEEL FRAMING MEMBERS Summary: The purpose of this document is to give engineers, architects, builders and home and commercial building owners a better understanding of how galvanizing (zinc and zinc alloy coatings) provides long-term corro- sion protection to cold-formed steel framing members. This document also suggests guidelines for selecting, han- dling and using these steels in framing applications. Disclaimer: Designs cited herein are not intended to preclude the use of other materials, assemblies, structures or designs when these other designs and materials demonstrate equivalent performance for the intended use; CFSEI documents are not intended to exclude the use and implementation of any other design or construction technique. 1.0 DESIGN LIFE and applications for zinc and zinc alloy coated steel sheet can be found on the GalvInfo Center web site at A home is one of the few necessities that consumers www.galvinfo.com. expect to last a lifetime or more. For commercial prop- erty owners, each structure represents a significant 2.2 Types of Coatings investment. It is critical therefore, that the framing ma- terial performs its function for as long as other critical The continuous galvanizing process can apply a num- components, such as the roof structure, exterior and ber of different coatings that vary in thickness, appear- interior wall coverings, and flooring. For sufficient lon- ance and alloy composition. A number of coating com- gevity, cold-formed steel framing needs proper corro- positions are acceptable for cold-formed steel framing, sion protection. -

Wet Storage Stain (White Rust)
Wet Storage Stain WET STORAGE STAIN galvanized coatings. (WHITE RUST) When this surface has access to freely moving air in normal atmospheric exposure, it reacts with rainfall Wet storage stain, also known as white rust, is the or dew to form a porous, gelatinous zinc hydroxide cor- voluminous white or gray deposit formed by accelerat- rosion product. During drying, this product reacts with ed corrosion of the zinc coating when closely-packed, carbon dioxide in the atmosphere and converts into a newly-galvanized articles are stored or shipped under thin, compact and tightly adherent layer of corrosion damp and poorly ventilated conditions. It is found most products consisting mainly of basic zinc carbonate gen- often on stacked and bundled items, such as galvanized erally written as 2ZnCO3·3Zn(OH)2. sheets, plates, angles, bars, and pipe. Weathered zinc The long life normally associated with galvanized surfaces which have already formed their normal pro- coatings in atmospheric service is entirely dependent tective layer of corrosion products are seldom attacked. upon the protection of the basic zinc carbonate. Being Due to their configuration, many products galvanized relatively insoluble, the basic zinc carbonate layer is after fabrication are less susceptible to wet storage stain- weather-resistant and, once formed, minimizes further ing than plain galvanized wire and sheet. corrosion. After a period of time, this whitish-gray Wet storage stain results from the exposure condi- powdery film tends to mask the underlying zinc crystals tions and is not indicative of inferior or poor quality gal- on the surface of the galvanized coating. vanizing. -

Galvinfonote Protecting Galvanized Steel Sheet Products from Storage
3. Corrosion – Mechanisms, Prevention, and Testing GalvInfoNote Protecting Galvanized Steel Sheet Products from Storage Stain 3.2 REV 2.1 DEC 2017 Introduction Storage stain, when related to galvanized sheet products, is a corrosion stain that is typically white, but which can also take the form of a grey or black deposit on the surface. Since the most common form of discoloration is white in appearance, storage stain is often called white rust. It can occur when sheets of galvanized steel that are in close contact (in a coil or stacked in lifts/bundles) get wet, either by direct water intrusion, or condensation between the surfaces. The discoloration is due to the corrosion products that form after zinc reacts with moisture in the absence of free air circulation. Building erected using roll-formed galvanized panels from a bundle that had extensive amounts of white rust. Note: This voluminous amount of white rust did not occur after erection of the building siding. When freely exposed to air, the products of zinc corrosion form a thin, tenacious film. The surface accumulation seen here occurred while the sheets were stored in a wet bundle. Before discussing the issue of storage stain in more detail, let’s first review what happens when a galvanized (zinc) coating corrodes in the environment. Why Does Zinc Protect Steel? Zinc, by its very nature, is a “reactive” metal and tends to corrode quite readily when exposed to moisture. Why then does it protect steel when a zinc-coated sheet is exposed to the atmosphere? When zinc corrodes in the presence of air and moisture it undergoes a series of chemical reactions, changing from metallic zinc on the surface to other chemical compounds. -

Protecting Galvanized Steel Sheet Products from Storage Stain
3. Corrosion – Mechanisms, Prevention, and Testing GalvInfoNote Protecting Galvanized Steel Sheet Products from Storage Stain 3.2 REV 3.0 OCT 2020 Introduction Storage stain, when related to galvanized sheet products, is a corrosion stain that is typically white, but which can also take the form of a grey or black deposit on the surface. Since the most common form of discoloration is white in appearance, storage stain is often called white rust. It can occur when sheets of galvanized steel that are in close contact (in a coil or stacked in lifts/bundles) get wet, either by direct water intrusion, or condensation between the surfaces. The discoloration is due to the corrosion products that form after zinc reacts with moisture in the absence of free air circulation. Building erected using roll-formed galvanized panels from a bundle that had extensive amounts of white rust. Note: This voluminous amount of white rust did not occur after erection of the building siding. When freely exposed to air, the products of zinc corrosion form a thin, tenacious film. The surface accumulation seen here occurred while the sheets were stored in a wet bundle. Before discussing the issue of storage stain in more detail, let’s first review what happens when a galvanized (zinc) coating corrodes in the environment. Why Does Zinc Protect Steel? Zinc, by its very nature, is a “reactive” metal and tends to corrode quite readily when exposed to moisture. Why then does it protect steel when a zinc-coated sheet is exposed to the atmosphere? When zinc corrodes in the presence of air and moisture it undergoes a series of chemical reactions, changing from metallic zinc on the surface to other chemical compounds.