Drug Eruptions: Great Imitators Chia-Yu Chu, MD, Phd⁎
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Neonatal Dermatology Review
NEONATAL Advanced Desert DERMATOLOGY Dermatology Jennifer Peterson Kevin Svancara Jonathan Bellew DISCLOSURES No relevant financial relationships to disclose Off-label use of acitretin in ichthyoses will be discussed PHYSIOLOGIC Vernix caseosa . Creamy biofilm . Present at birth . Opsonizing, antibacterial, antifungal, antiparasitic activity Cutis marmorata . Reticular, blanchable vascular mottling on extremities > trunk/face . Response to cold . Disappears on re-warming . Associations (if persistent) . Down syndrome . Trisomy 18 . Cornelia de Lange syndrome PHYSIOLOGIC Milia . Hard palate – Bohn’s nodules . Oral mucosa – Epstein pearls . Associations . Bazex-Dupre-Christol syndrome (XLD) . BCCs, follicular atrophoderma, hypohidrosis, hypotrichosis . Rombo syndrome . BCCs, vermiculate atrophoderma, trichoepitheliomas . Oro-facial-digital syndrome (type 1, XLD) . Basal cell nevus (Gorlin) syndrome . Brooke-Spiegler syndrome . Pachyonychia congenita type II (Jackson-Lawler) . Atrichia with papular lesions . Down syndrome . Secondary . Porphyria cutanea tarda . Epidermolysis bullosa TRANSIENT, NON-INFECTIOUS Transient neonatal pustular melanosis . Birth . Pustules hyperpigmented macules with collarette of scale . Resolve within 4 weeks . Neutrophils Erythema toxicum neonatorum . Full term . 24-48 hours . Erythematous macules, papules, pustules, wheals . Eosinophils Neonatal acne (neonatal cephalic pustulosis) . First 30 days . Malassezia globosa & sympoidalis overgrowth TRANSIENT, NON-INFECTIOUS Miliaria . First weeks . Eccrine -

An Atypical Case of Warfarin-Induced Skin Necrosis
CASE REPORT An Atypical Case of Warfarin-Induced Skin Necrosis Lindsay R. Sklar, MD* *Wayne State University, Department of Dermatology, Dearborn, Michigan Anne Messman, MD, FACEP† †Sinai-Grace Hospital, Department of Emergency Medicine, Detroit, Michigan Section Editor: Rick A. McPheeters, DO Submission history: Submitted December 19, 2016; Revision received March 8, 2017; Accepted March 30, 2017 Electronically published October 11, 2017 Full text available through open access at http://escholarship.org/uc/uciem_cpcem DOI: 10.5811/cpcem.2017.3.33373 Skin necrosis is a relatively rare, potentially fatal side effect of warfarin. It is most commonly reported within 10 days of initiation of therapy in warfarin-naïve patients. We report an atypical case of warfarin-induced skin necrosis upon recommencement of warfarin in a non-naïve warfarin patient. [Clin Pract Cases Emerg Med. 2017;1(4):359–361.] INTRODUCTION were significant for a normal platelet level of 180,000/mm,³ Warfarin is currently the most widely prescribed oral partial thromboplastin time of 27.7 sec, prothrombin time of anticoagulant in North America. Thrombosis is a rare, 21.8 sec, and an international normalized ratio (INR) of 1.87. paradoxical, and potentially fatal adverse effect of the drug. Computerized tomography (CT) of the abdomen with and Skin necrosis occurs secondary to the development of without intravenous contrast showed no evidence of microthrombi and endothelial cell damage in the vessels of malignancy or hemorrhage. dermal and subcutaneous tissues. Since this rare The patient had been on the same warfarin regimen (7.5mg complication was first recognized in 1943, there have been three days a week and 10mg four days a week) for two years an estimated 300 cases reported, affecting approximately prior to presentation to our ED; it had been initiated after the 0.01-0.1% of patients on the drug.1 Typically, warfarin- development of a deep vein thrombosis and pulmonary induced skin necrosis presents within three to ten days of embolism. -

Seborrheic Dermatitis: an Overview ROBERT A
Seborrheic Dermatitis: An Overview ROBERT A. SCHWARTZ, M.D., M.P.H., CHRISTOPHER A. JANUSZ, M.D., and CAMILA K. JANNIGER, M.D. University of Medicine and Dentistry at New Jersey-New Jersey Medical School, Newark, New Jersey Seborrheic dermatitis affects the scalp, central face, and anterior chest. In adolescents and adults, it often presents as scalp scaling (dandruff). Seborrheic dermatitis also may cause mild to marked erythema of the nasolabial fold, often with scaling. Stress can cause flare-ups. The scales are greasy, not dry, as commonly thought. An uncommon generalized form in infants may be linked to immunodeficiencies. Topical therapy primarily consists of antifungal agents and low-potency steroids. New topical calcineurin inhibitors (immunomodulators) sometimes are administered. (Am Fam Physician 2006;74:125-30. Copyright © 2006 American Academy of Family Physicians.) eborrheic dermatitis can affect patients levels, fungal infections, nutritional deficits, from infancy to old age.1-3 The con- neurogenic factors) are associated with the dition most commonly occurs in condition. The possible hormonal link may infants within the first three months explain why the condition appears in infancy, S of life and in adults at 30 to 60 years of age. In disappears spontaneously, then reappears adolescents and adults, it usually presents as more prominently after puberty. A more scalp scaling (dandruff) or as mild to marked causal link seems to exist between seborrheic erythema of the nasolabial fold during times dermatitis and the proliferation of Malassezia of stress or sleep deprivation. The latter type species (e.g., Malassezia furfur, Malassezia tends to affect men more often than women ovalis) found in normal dimorphic human and often is precipitated by emotional stress. -

Dermatology DDX Deck, 2Nd Edition 65
63. Herpes simplex (cold sores, fever blisters) PREMALIGNANT AND MALIGNANT NON- 64. Varicella (chicken pox) MELANOMA SKIN TUMORS Dermatology DDX Deck, 2nd Edition 65. Herpes zoster (shingles) 126. Basal cell carcinoma 66. Hand, foot, and mouth disease 127. Actinic keratosis TOPICAL THERAPY 128. Squamous cell carcinoma 1. Basic principles of treatment FUNGAL INFECTIONS 129. Bowen disease 2. Topical corticosteroids 67. Candidiasis (moniliasis) 130. Leukoplakia 68. Candidal balanitis 131. Cutaneous T-cell lymphoma ECZEMA 69. Candidiasis (diaper dermatitis) 132. Paget disease of the breast 3. Acute eczematous inflammation 70. Candidiasis of large skin folds (candidal 133. Extramammary Paget disease 4. Rhus dermatitis (poison ivy, poison oak, intertrigo) 134. Cutaneous metastasis poison sumac) 71. Tinea versicolor 5. Subacute eczematous inflammation 72. Tinea of the nails NEVI AND MALIGNANT MELANOMA 6. Chronic eczematous inflammation 73. Angular cheilitis 135. Nevi, melanocytic nevi, moles 7. Lichen simplex chronicus 74. Cutaneous fungal infections (tinea) 136. Atypical mole syndrome (dysplastic nevus 8. Hand eczema 75. Tinea of the foot syndrome) 9. Asteatotic eczema 76. Tinea of the groin 137. Malignant melanoma, lentigo maligna 10. Chapped, fissured feet 77. Tinea of the body 138. Melanoma mimics 11. Allergic contact dermatitis 78. Tinea of the hand 139. Congenital melanocytic nevi 12. Irritant contact dermatitis 79. Tinea incognito 13. Fingertip eczema 80. Tinea of the scalp VASCULAR TUMORS AND MALFORMATIONS 14. Keratolysis exfoliativa 81. Tinea of the beard 140. Hemangiomas of infancy 15. Nummular eczema 141. Vascular malformations 16. Pompholyx EXANTHEMS AND DRUG REACTIONS 142. Cherry angioma 17. Prurigo nodularis 82. Non-specific viral rash 143. Angiokeratoma 18. Stasis dermatitis 83. -

Pompholyx Factsheet Pompholyx Eczema (Also Known As Dyshidrotic Eczema/Dermatitis) Is a Type of Eczema That Usually Affects the Hands and Feet
12 Pompholyx factsheet Pompholyx eczema (also known as dyshidrotic eczema/dermatitis) is a type of eczema that usually affects the hands and feet. In most cases, pompholyx eczema involves the development of intensely itchy, watery blisters, mostly affecting the sides of the fingers, the palms of the hands and soles of the feet. Some people have pompholyx eczema on their hands and/or feet with other types of eczema elsewhere on the body. This condition can occur at any age but is usually seen in adults under 40, and is more common in women. The skin is initially very itchy with a burning sensation of heat and prickling in the palms and/or soles. Then comes a sudden crop of small blisters (vesicles), which turn into bigger weepy blisters, which can become infected, causing redness, pain, swelling and pustules. There is often subsequent peeling as the skin dries out, and then the skin can become red and dry with painful cracks (skin fissures). Pompholyx eczema can also affect the nail folds and skin around the nails, causing swelling (paronychia). What causes it? A reaction could be the result of contact with potential irritants such as soap, detergents, solvents, acids/alkalis, The exact causes of pompholyx eczema are not known, chemicals and soil, causing irritant contact dermatitis. Or although it is thought that factors such as stress, there could be an allergic reaction to a substance that is sensitivity to metal compounds (such as nickel, cobalt or not commonly regarded as an irritant, such as rubber or chromate), heat and sweating can aggravate this nickel, causing allergic contact dermatitis. -

Cutaneous Adverse Effects of Biologic Medications
REVIEW CME MOC Selena R. Pasadyn, BA Daniel Knabel, MD Anthony P. Fernandez, MD, PhD Christine B. Warren, MD, MS Cleveland Clinic Lerner College Department of Pathology Co-Medical Director of Continuing Medical Education; Department of Dermatology, Cleveland Clinic; of Medicine of Case Western and Department of Dermatology, W.D. Steck Chair of Clinical Dermatology; Director of Clinical Assistant Professor, Cleveland Clinic Reserve University, Cleveland, OH Cleveland Clinic Medical and Inpatient Dermatology; Departments of Lerner College of Medicine of Case Western Dermatology and Pathology, Cleveland Clinic; Assistant Reserve University, Cleveland, OH Clinical Professor, Cleveland Clinic Lerner College of Medicine of Case Western Reserve University, Cleveland, OH Cutaneous adverse effects of biologic medications ABSTRACT iologic therapy encompasses an expo- B nentially expanding arena of medicine. Biologic therapies have become widely used but often As the name implies, biologic therapies are de- cause cutaneous adverse effects. The authors discuss the rived from living organisms and consist largely cutaneous adverse effects of tumor necrosis factor (TNF) of proteins, sugars, and nucleic acids. A clas- alpha inhibitors, epidermal growth factor receptor (EGFR) sic example of an early biologic medication is inhibitors, small-molecule tyrosine kinase inhibitors insulin. These therapies have revolutionized (TKIs), and cell surface-targeted monoclonal antibodies, medicine and offer targeted therapy for an including how to manage these reactions -
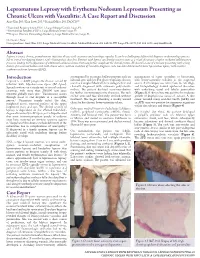
Lepromatous Leprosy with Erythema Nodosum Leprosum Presenting As
Lepromatous Leprosy with Erythema Nodosum Leprosum Presenting as Chronic Ulcers with Vasculitis: A Case Report and Discussion Anny Xiao, DO,* Erin Lowe, DO,** Richard Miller, DO, FAOCD*** *Traditional Rotating Intern, PGY-1, Largo Medical Center, Largo, FL **Dermatology Resident, PGY-2, Largo Medical Center, Largo, FL ***Program Director, Dermatology Residency, Largo Medical Center, Largo, FL Disclosures: None Correspondence: Anny Xiao, DO; Largo Medical Center, Graduate Medical Education, 201 14th St. SW, Largo, FL 33770; 510-684-4190; [email protected] Abstract Leprosy is a rare, chronic, granulomatous infectious disease with cutaneous and neurologic sequelae. It can be a challenging differential diagnosis in dermatology practice due to several overlapping features with rheumatologic disorders. Patients with leprosy can develop reactive states as a result of immune complex-mediated inflammatory processes, leading to the appearance of additional cutaneous lesions that may further complicate the clinical picture. We describe a case of a woman presenting with a long history of a recurrent bullous rash with chronic ulcers, with an evolution of vasculitic diagnoses, who was later determined to have lepromatous leprosy with reactive erythema nodosum leprosum (ENL). Introduction accompanied by an intense bullous purpuric rash on management of sepsis secondary to bacteremia, Leprosy is a slowly progressive disease caused by bilateral arms and face. For these complaints she was with lower-extremity cellulitis as the suspected infection with Mycobacterium leprae (M. leprae). seen in a Complex Medical Dermatology Clinic and source. A skin biopsy was taken from the left thigh, Spread continues at a steady rate in several endemic clinically diagnosed with cutaneous polyarteritis and histopathology showed epidermal ulceration countries, with more than 200,000 new cases nodosa. -

ORIGINAL ARTICLE a Clinical and Histopathological Study of Lichenoid Eruption of Skin in Two Tertiary Care Hospitals of Dhaka
ORIGINAL ARTICLE A Clinical and Histopathological study of Lichenoid Eruption of Skin in Two Tertiary Care Hospitals of Dhaka. Khaled A1, Banu SG 2, Kamal M 3, Manzoor J 4, Nasir TA 5 Introduction studies from other countries. Skin diseases manifested by lichenoid eruption, With this background, this present study was is common in our country. Patients usually undertaken to know the clinical and attend the skin disease clinic in advanced stage histopathological pattern of lichenoid eruption, of disease because of improper treatment due to age and sex distribution of the diseases and to difficulties in differentiation of myriads of well assess the clinical diagnostic accuracy by established diseases which present as lichenoid histopathology. eruption. When we call a clinical eruption lichenoid, we Materials and Method usually mean it resembles lichen planus1, the A total of 134 cases were included in this study prototype of this group of disease. The term and these cases were collected from lichenoid used clinically to describe a flat Bangabandhu Sheikh Mujib Medical University topped, shiny papular eruption resembling 2 (Jan 2003 to Feb 2005) and Apollo Hospitals lichen planus. Histopathologically these Dhaka (Oct 2006 to May 2008), both of these are diseases show lichenoid tissue reaction. The large tertiary care hospitals in Dhaka. Biopsy lichenoid tissue reaction is characterized by specimen from patients of all age group having epidermal basal cell damage that is intimately lichenoid eruption was included in this study. associated with massive infiltration of T cells in 3 Detailed clinical history including age, sex, upper dermis. distribution of lesions, presence of itching, The spectrum of clinical diseases related to exacerbating factors, drug history, family history lichenoid tissue reaction is wider and usually and any systemic manifestation were noted. -

Erythema Annulare Centrifugum ▪ Erythema Gyratum Repens ▪ Exfoliative Erythroderma Urticaria ▪ COMMON: 15% All Americans
Cutaneous Signs of Internal Malignancy Ted Rosen, MD Professor of Dermatology Baylor College of Medicine Disclosure/Conflict of Interest ▪ No relevant disclosures ▪ No conflicts of interest Objectives ▪ Recognize common disorders associated with internal malignancy ▪ Manage cutaneous disorders in the context of associated internal malignancy ▪ Differentiate cutaneous signs of leukemia and lymphoma ▪ Understand spidemiology of cutaneous metastases Cutaneous Signs of Internal Malignancy ▪ General physical examination ▪ Pallor (anemia) ▪ Jaundice (hepatic or cholestatic disease) ▪ Fixed erythema or flushing (carcinoid) ▪ Alopecia (diffuse metastatic disease) ▪ Itching (excoriations) Anemia: Conjunctival pallor and Pale skin Jaundice 1-12% of hepatocellular, biliary tree or pancreatic cancer PRESENT with jaundice, but up to 40-60% eventually develop it World J Gastroenterol 2003;9:385-91 For comparison CAN YOU TELL JAUNDICE FROM NORMAL SKIN? JAUNDICE Alopecia Neoplastica Most common report w/ breast CA Lung, cervix, desmoplastic mm Hair loss w/ underlying induration Biopsy = dermis effaced by tumor Ann Dermatol 26:624, 2014 South Med J 102:385, 2009 Int J Dermatol 46:188, 2007 Acta Derm Venereol 87:93, 2007 J Eur Acad Derm Venereol 18:708, 2004 Gastric Adenocarcinoma: Alopecia Ann Dermatol 2014; 26: 624–627 Pruritus: Excoriation ▪ Overall risk internal malignancy presenting as itch LOW. OR =1.14 ▪ CTCL, Hodgkin’s & NHL, Polycythemia vera ▪ Biliary tree carcinoma Eur J Pain 20:19-23, 2016 Br J Dermatol 171:839-46, 2014 J Am Acad Dermatol 70:651-8, 2014 Non-specific (Paraneoplastic) Specific (Metastatic Disease) Paraneoplastic Signs “Curth’s Postulates” ▪ Concurrent onset (temporal proximity) ▪ Parallel course ▪ Uniform site or type of neoplasm ▪ Statistical association ▪ Genetic linkage (syndromal) Curth HO. -
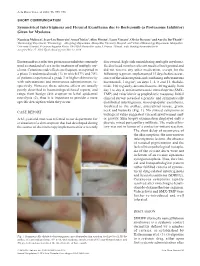
Symmetrical Intertriginous and Flexural Exanthema Due to Bortezomib (A Proteasome Inhibitor) Given for Myeloma
Acta Derm Venereol 2016; 96: 995–996 SHORT COMMUNICATION Symmetrical Intertriginous and Flexural Exanthema due to Bortezomib (a Proteasome Inhibitor) Given for Myeloma Nausicaa Malissen1, Jean-Luc Bourrain1, Anca Chiriac2, Aline Montet1, Laure Vincent3, Olivier Dereure1 and Aurélie Du-Thanh1* 1Dermatology Department, 2Pneumology – Allergology Department, Montpellier University Hospital, and 3Clinical Hematology Department, Montpellier University Hospital, 80 avenue Augustin Fliche, FR-34295 Montpellier cedex 5, France. *E-mail: [email protected] Accepted Mar 17, 2016; Epub ahead of print Mar 22, 2016 Bortezomib is a selective proteasome inhibitor currently discovered, high-risk smouldering multiple myeloma. used as standard of care in the treatment of multiple my- He disclosed no other relevant medical background and eloma. Cutaneous side-effects are frequent, as reported in did not receive any other medication, except for the a phase 3 randomized study (1), in which 57% and 70% following regimen, implemented 15 days before occur- of patients experienced a grade 3 or higher skin toxicity rence of the skin eruption and combining subcutaneous with subcutaneous and intravenous administration, re- bortezomib, 1 mg/m2, on days 1, 4, 8 and 11, thalido- spectively. However, these adverse effects are usually mide, 100 mg daily, dexamethasone, 40 mg daily from poorly described in haematological-based reports, and day 1 to day 4, sulfamethoxazole trimethoprim (SMX- range from benign skin eruption to lethal epidermal TMP) and valaciclovir as prophylactic measures. Initial necrolysis (2), thus it is important to provide a more clinical survey revealed a pruritic and symmetrically specific description when they occur. distributed intertriginous, maculopapular exanthema, localized to the axillae, antecubital fossae, groin, neck and buttocks (Fig. -

Alopecia, Particularly: Alopecia Areata Androgenetic Alopecia Telogen Effluvium Anagen Effluvium
432 Teams Dermatology Hair disorders Color Code: Original, Team’s note, Important, Doctor’s note, Not important, Old teamwork Done by: Shaikha Aldossari Reviewer: Lama AlTawil 8 Team Leader: Basil Al Suwaine&Lama Al Tawil 432 Dermatology Team Lecture 8: Hair Disorders Objectives 1- Normal anatomy of hair follicle and hair cycle. 2- Causes, features and management of non scarring alopecia, particularly: Alopecia areata Androgenetic alopecia Telogen effluvium Anagen effluvium 3- Causes and features of scarring alopecia. 4- Causes and features of Excessive hair growth. hair disorder Excessive hair Alopecia growth non scarring Hirsutism Hypertrichosis scarring Anagen Telogen Androgenetic Alopecia effluvium effluvium Alopecia Areata P a g e | 1 432 Dermatology Team Lecture 8: Hair Disorders Anatomy of hair follicle: The Arrector piliResponsible for piloerection (goose bumps ) that happens when one is cold (produces energy and therefor warmth) . hair follicle becomes vertical instead of oblique Cuticle is the last layer here . what we can see outside . it has 7 layers of keratinocytes How many hairs in the body? 5 millions hairs in the body, 100,000 in the scalp. Growth rate: 0.3mm/day for scalp hair i.e.1cm/month Hair follicle bulge: -Very important part since it has stem cells .its the inertion of the arrector pili Hair follicle on vertical section: -So any pathological process affecting any part other Initially the shaft and the follicle are one than this, hair would still be able to regrow. organ then when you reach 1/3 the follicle -If we want to destroy a hair follicle, we’d target the bulge. -

Fundamentals of Dermatology Describing Rashes and Lesions
Dermatology for the Non-Dermatologist May 30 – June 3, 2018 - 1 - Fundamentals of Dermatology Describing Rashes and Lesions History remains ESSENTIAL to establish diagnosis – duration, treatments, prior history of skin conditions, drug use, systemic illness, etc., etc. Historical characteristics of lesions and rashes are also key elements of the description. Painful vs. painless? Pruritic? Burning sensation? Key descriptive elements – 1- definition and morphology of the lesion, 2- location and the extent of the disease. DEFINITIONS: Atrophy: Thinning of the epidermis and/or dermis causing a shiny appearance or fine wrinkling and/or depression of the skin (common causes: steroids, sudden weight gain, “stretch marks”) Bulla: Circumscribed superficial collection of fluid below or within the epidermis > 5mm (if <5mm vesicle), may be formed by the coalescence of vesicles (blister) Burrow: A linear, “threadlike” elevation of the skin, typically a few millimeters long. (scabies) Comedo: A plugged sebaceous follicle, such as closed (whitehead) & open comedones (blackhead) in acne Crust: Dried residue of serum, blood or pus (scab) Cyst: A circumscribed, usually slightly compressible, round, walled lesion, below the epidermis, may be filled with fluid or semi-solid material (sebaceous cyst, cystic acne) Dermatitis: nonspecific term for inflammation of the skin (many possible causes); may be a specific condition, e.g. atopic dermatitis Eczema: a generic term for acute or chronic inflammatory conditions of the skin. Typically appears erythematous,