CTRI Trial Data
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Details of Vehicle Dealers
DETAILS OF VEHICLE DEALERS (BOTH SELF AND NON-SELF) AS ON 17.09.2020 BASED ON VAHAN DATABASE S NO RTO Code Dealer Name Address Maker Self/Non-self Vehicle Class 1 1 RANA MOTORS P.LTD NA DL-110054 MARUTI SUZUKI INDIA LTD Self Motor Car 2 1 M/S PAHWA AUTOSALES PVT LTD 26/24,26/25 SHAKTI NAGAR DL-110007 HYUNDAI MOTOR INDIA LTD Non-self Motor Car 3 1 BALAJI AUTO 31, SHAHZAD BAGH INDL AREA DELHI DL-110035 NA Non-self M-Cycle/Scooter 1275-1276 BARA BAZAR KASMERE GATE DELHI DL- 4 1 CARLTON MOTORS PVT LTD NA Non-self M-Cycle/Scooter 110006 5 1 RANA MOTORS NA DL-110054 MARUTI SUZUKI INDIA LTD Self Motor Car 6 2 Apra Auto India Pvt Ltd NA New Delhi DL- MARUTI SUZUKI INDIA LTD Self Motor Car 7 2 COMPETENT AUTOMOBILES CO. LTD. COMPETENT HOUSE, F-14, CP NEW DELHI DL-110001 NA Self Motor Car RIGH ROAD HONDA,40- 42 JANPATH, NEW DELHI DL- 8 2 BAS ENGINEER PVT. LIMITED HONDA CARS INDIA LTD Non-self Motor Car 110001 CAPITAL POINT, BABA KHARAT SINGH MARG, 9 2 ZENICA CARS INDIA PVT. LIMITED AUDI AG Non-self Motor Car CONNAUGHT PLACE DL-110001 HOTEL SAMRAT, LGF, KAUTILYA MARG, CHANAKYA 10 2 EXCLUSIVE MOTORS PVT. LIMITED BENTLEY MOTORS LIMITED Non-self Motor Car PURI DL-110001 HOTEL SAMRAT, LGF, KAUTILYA MARG CHANAKYA 11 2 EXCLUSIVE MOTORS PVT. LTD NA Non-self Motor Car PURI DL-110001 12 2 SILVER ARROW 50-B,CHANAKAYA PURI DL-110001 NA Non-self Motor Car 13 2 COMPETENT AUTOMOBILES CO. -

NDMC Ward No. 001 S
NDMC Ward No. 001 S. No. Ward Name of Name of Name of Enumeratio Extent of the Population Enumeration Total SC % of SC Name & town/Census District & Tahsil & n Block No. Block Population Population Population Code Town/ Village Code Code 0001 NDMC 7003 New Delhi 05 Connaught 0021(1) Devi Prasad Sadan 1-64, NDMC Flats 4 Place 001 Type-6, Asha Deep Apartment 9 Hailey 1 Road 44 Flats 656 487 74.24 0001 NDMC 7003 New Delhi 05 Connaught 0029 Sangli Mess Cluster (Slum) 2 Place 001 351 174 49.57 0001 NDMC 7003 New Delhi 05 Connaught 0031(2) Feroz Shah Road, Canning Lane Kerala 3 Place 001 School 593 212 35.75 0001 NDMC 7003 New Delhi 05 Connaught 0032(1) Princess Park Residential Area Copper 4 Place 001 Nicus Marg to Tilak Marg, 100 Houses 276 154 55.8 0001 NDMC 7003 New Delhi 05 Connaught 0032(2) Princess Park Residential Area Copper 5 Place 001 Nicus Marg to Tilak Marg, 105 Houses 312 142 45.51 0001 NDMC 7003 New Delhi 05 Connaught 0036(1) NSCI Club Cluster-171 Houses 6 Place 001 521 226 43.38 NDMC Ward No. 002 Ward Name of Name of Name of Enumeratio Extent of the Population Enumeration Total SC % of SC Name & town/Census District & Tahsil & n Block No. Block Population Population Population S. No. Code Town/ Village Code Code Parliament A1 to H18 CN 1 to 10 Palika Dham Bhai Vir 0002 NDMC 7003 New Delhi 05 0005-1 933 826 88.53 1 Street 003 Singh Marg Block 5 Jain Mandir Marg ,Vidhya Bhawan Parliament 0002 NDMC 7003 New Delhi 05 0009 ,Union Acadmy Colony 70 A -81 H Arya 585 208 35.56 Street 003 2 School Lane Parliament 1-126 Mandir Marg R.K. -

CONTACT LIST CGHS DELHI-ADDITIONAL DIRECTOR OFFICES CONTACT DETAILS for OFFICE of ADDITIONAL DIRECTOR CGHS HEADQUARTER, DELHI Contact S.N
CONTACT LIST CGHS DELHI-ADDITIONAL DIRECTOR OFFICES CONTACT DETAILS FOR OFFICE OF ADDITIONAL DIRECTOR CGHS HEADQUARTER, DELHI Contact S.N. Official post Name of the Officer Address number (area Official e-mail ID code 011) 1 Additional Director Dr Pardeep Kumar O/O Additional Director CGHS (HQ), CGHS Wellness 26712279, [email protected] (HQ) Centre Building Sector-12 R.K Puram New Delhi- 26712280 110022 2 DDO Shri H S Gupta O/O Additional Director CGHS (HQ), CGHS Wellness 26175601 [email protected] Centre Building Sector-12 R.K Puram New Delhi- 110022 3 Nodal Officer (RTI) Dr Mohan Lal O/O Additional Director CGHS (HQ), CGHS Wellness 26712276 [email protected] Centre Building Sector-12 R.K Puram New Delhi- 110022 4 Joint Director (HQ) Dr Sanjai Jain O/O Additional Director CGHS (HQ), CGHS Wellness 26712267 [email protected] Centre Building Sector-12 R.K Puram New Delhi- 110022 5 Joint Director(R&H) Dr V Sushash O/O Additional Director CGHS (HQ), CGHS Wellness 26712278 [email protected] Centre Building Sector-12 R.K Puram New Delhi- 110022 6 Joint Dr Rupak Chatterjee O/O Additional Director CGHS (HQ), CGHS Wellness 26173164 [email protected] Director(Grievance) Centre Building Sector-12 R.K Puram New Delhi- 110022 7 ADO(NG) Sh Sandeep sharma O/O Additional Director CGHS (HQ), CGHS Wellness 26712269 [email protected] Centre Building Sector-12 R.K Puram New Delhi- 110022 8 CMO(Hospital Cell) Dr Subhash O/O Additional Director CGHS (HQ), CGHS Wellness 26175604 [email protected] Centre Building Sector-12 R.K Puram -

EMU 64087 Train Time Schedule & Line Route
EMU 64087 train time schedule & line map EMU 64087 Hazrat Nizamuddin (NZM) -New Delhi View In Website Mode (NDLS) Parikrama The EMU 64087 train line Hazrat Nizamuddin (NZM) -New Delhi (NDLS) Parikrama has one route. For regular weekdays, their operation hours are: (1) New Delhi (Ndls): 6:27 PM Use the Moovit App to ƒnd the closest EMU 64087 train station near you and ƒnd out when is the next EMU 64087 train arriving. Direction: New Delhi (Ndls) EMU 64087 train Time Schedule 17 stops New Delhi (Ndls) Route Timetable: VIEW LINE SCHEDULE Sunday Not Operational Monday Not Operational Hazrat Nizamuddin Tuesday Not Operational Lajpat Nagar Wednesday Not Operational Sewa Nagar Thursday Not Operational Lodhi Colony Friday 6:27 PM Sarojini Nagar Saturday Not Operational Delhi Safdarjung Chanakyapuri EMU 64087 train Info Sardar Patel Marg Direction: New Delhi (Ndls) Stops: 17 Trip Duration: 43 min Brar Square Line Summary: Hazrat Nizamuddin, Lajpat Nagar, Sewa Nagar, Lodhi Colony, Sarojini Nagar, Delhi Indrapuri Safdarjung, Chanakyapuri, Sardar Patel Marg, Brar Square, Indrapuri, Naraina Vihar, Kirti Nagar, Patel Naraina Vihar Nagar, Dayabasti, Vivekanand Puri Halt, Kishanganj, New Delhi Kirti Nagar Patel Nagar Dayabasti Vivekanand Puri Halt Kishanganj New Delhi EMU 64087 train time schedules and route maps are available in an o«ine PDF at moovitapp.com. Use the Moovit App to see live bus times, train schedule or subway schedule, and step-by-step directions for all public transit in New Delhi. Check Live Arrival Times About Moovit MaaS Solutions Supported Countries Mooviter Community © 2021 Moovit - All Rights Reserved. -

Government Cvcs for Covid Vaccination for 18 Years+ Population
S.No. District Name CVC Name 1 Central Delhi Anglo Arabic SeniorAjmeri Gate 2 Central Delhi Aruna Asaf Ali Hospital DH 3 Central Delhi Balak Ram Hospital 4 Central Delhi Burari Hospital 5 Central Delhi CGHS CG Road PHC 6 Central Delhi CGHS Dev Nagar PHC 7 Central Delhi CGHS Dispensary Minto Road PHC 8 Central Delhi CGHS Dispensary Subzi Mandi 9 Central Delhi CGHS Paharganj PHC 10 Central Delhi CGHS Pusa Road PHC 11 Central Delhi Dr. N.C. Joshi Hospital 12 Central Delhi ESI Chuna Mandi Paharganj PHC 13 Central Delhi ESI Dispensary Shastri Nagar 14 Central Delhi G.B.Pant Hospital DH 15 Central Delhi GBSSS KAMLA MARKET 16 Central Delhi GBSSS Ramjas Lane Karol Bagh 17 Central Delhi GBSSS SHAKTI NAGAR 18 Central Delhi GGSS DEPUTY GANJ 19 Central Delhi Girdhari Lal 20 Central Delhi GSBV BURARI 21 Central Delhi Hindu Rao Hosl DH 22 Central Delhi Kasturba Hospital DH 23 Central Delhi Lady Reading Health School PHC 24 Central Delhi Lala Duli Chand Polyclinic 25 Central Delhi LNJP Hospital DH 26 Central Delhi MAIDS 27 Central Delhi MAMC 28 Central Delhi MCD PRI. SCHOOl TRUKMAAN GATE 29 Central Delhi MCD SCHOOL ARUNA NAGAR 30 Central Delhi MCW Bagh Kare Khan PHC 31 Central Delhi MCW Burari PHC 32 Central Delhi MCW Ghanta Ghar PHC 33 Central Delhi MCW Kanchan Puri PHC 34 Central Delhi MCW Nabi Karim PHC 35 Central Delhi MCW Old Rajinder Nagar PHC 36 Central Delhi MH Kamla Nehru CHC 37 Central Delhi MH Shakti Nagar CHC 38 Central Delhi NIGAM PRATIBHA V KAMLA NAGAR 39 Central Delhi Polyclinic Timarpur PHC 40 Central Delhi S.S Jain KP Chandani Chowk 41 Central Delhi S.S.V Burari Polyclinic 42 Central Delhi SalwanSr Sec Sch. -

LIST of Ngos & ADVOCATES
LIST OF NGOs & ADVOCATES Telephone No. S.N State Name of contact person & address STD Code Land line Fax Mobile 1 DELHI & Dr. (Ms.)Jyotsna Chatterjee, 0120 42143810 9810017523 NCR Director, Joint Women’s Programme, Flat 301, Shri Ram Residence Ahinsa Khand- 2 Indirapuram, Gaziabad [email protected] 2 DELHI & Ms. Indira Jaisingh 011 24373904 NCR Lawyers Collective 63/2, Masjid Road 1 st Floor, Jangpura Delhi – 110014 3 DELHI & Dr.(Smt.) Mohini Giri 011 NCR Guild of Services “SHUBHAM” C-25, Qutab Institutional Area, New Delhi Guild Of Service, Ms. Pallavi Tomar 4 DELHI & Ms. Ruchira Gupta 011 24312923 24110056 NCR Executive Director Apne Aap Women Worldwide 24313904 D-56, Anand Niketan, 4601 5940 New Delhi-110 021. email: 24110056 ruchira [email protected] 5 DELHI & Mr. Colin Gonsalves 011 24374501 NCR Advocate Human Rights Law Network 576,Masjid Road Jungpura,New Delhi14 [email protected] 6 Women Power Connect 011 42705170/ 9811301315 A1/125,1 st floor,Safdarjung Enclave 71/72 NewDelhi-29 [email protected] wrong 7 DELHI & Ms. Naina Kapoor 011 4623295 4643946 NCR Sakshi B-67, South Extension Part - I, 4643946 New Delhi – 110049 Email : [email protected] wrong 8 DELHI & Ms. Roma Debabrata 011 6425811 6425812 NCR President STOP A – 25, Ground floor Chitranjan Park New Delhi-110 019 [email protected] 9 DELHI & Ms.Sudha Sundaraman 011 23710476 9868525068 NCR AIDWA 121,VP House 23319566 Rafi Marg New Delhi Email: [email protected] Website: www.aidwa.org 10 DELHI & Ms.Madhu Mehra 011 24316832 NCR Executive Director Partners for Law and Development F-18, 1 st Floor Jangpura Extension, New Delhi-14 [email protected] 11 DELHI & Ms. -

Total No. of Diesel Vehicles Registered in SARAI
Total No. of Diesel Vehicles is registered before 07-nov-2001 or 15 years old and not have valid fitness on 08-nov-2016 Sno regn_no regn_dt fit_upto owner_name f_name p_add1 p_add2 p_add3 p_pincode descr off_name 45004 DDA9444 10-07-1987 09-07-2002 RAVI SHARMA N C SHARMA A-169 PREET VIHAR NEW DELHI 110092 . 0 DIESEL SARAI KALE KHAN 45005 DAQ5575 06-08-1984 05-08-1999 TARIKA SINGH D/O DARSHAN SINGH G 12 ANAND NIKETAN NEW DELHI . 0 DIESEL SARAI KALE KHAN 45006 DAQ5578 10-02-1989 09-02-2004 RAJESH AGGARWAL R AGGARWAL 16/7 SHAKTI NAGAR DELHI 110007 0 DIESEL SARAI KALE KHAN 45007 DAQ5579 10-02-1989 26-08-2009 S GURUMUKH SINGH S MOHINDER SINGH 157 ARJUN NAGAR NEW DELHI . 0 DIESEL SARAI KALE KHAN 45008 DAQ5581 10-02-1989 09-02-2004 M L DESHMUKH L R DESHMUKH SANT SAMAGAM 24 PARK AREA K BAGH NEW DELHI 110005 0 DIESEL SARAI KALE KHAN 45009 DDA0705 19-08-1987 18-08-2002 SH KUMAR JITENDER SINGH SIO THALAN DEVENDAR 5-DUPLEX ROAD NEW DELHI-110001 . 0 DIESEL SARAI KALE KHAN 45010 DAQ0155 12-10-1988 11-10-2003 RAMKISHAN S/O MOHAN LAL 51 APANA BAZAR NEHRU NAGAR DELHI . 0 DIESEL SARAI KALE KHAN 45011 DAQ0230 20-02-1989 19-02-2004 NARENDER G VACHANI S/O GAIN T VACHANI B-8 MAYFAIR GARDEN MG NEW DELHI-16 0 DIESEL SARAI KALE KHAN 45012 DAQ0353 24-11-1988 23-11-2003 SUKHWANT SINGH B SINGH D1/2491 JANAK PURI NEW DELHI 110058 0 DIESEL SARAI KALE KHAN 45013 DDA0713 19-08-1987 18-08-2002 INDARJEET SINGH JOGINDAR SINGH GALI NO-6 HOUSE 7967 MUZATANI PAHAR GANG NEW DELHI 0 DIESEL SARAI KALE KHAN 45014 DDA0715 19-08-1987 18-08-2002 RAKESH KUMAR RAM KUMAR 310 JOGINADER -

Dr.B.C.Rathore Cause List
011-28031838 011-28032406 Fax no. 28032381 Patents/Designs/Trademark GEOGRAPHICAL INDICATIONS GOVERNMENT OF INDIA MINISTRY OF COMMERCE & INDUSTRY Plot No.32, Sector – 14, Dwarka NEW DELHI - 110078. Name of Hearing Officer: DR.B.C.RATHORE (JOINT REGISTRAR) Date: 01/08/2019 Time: 11:00 AM. CAUSE LIST S.No OPPOSITION APPLICATION NAME OF PARTIES NAME OF REPRESENTATIVE. NO. NO. 1 174531 1091937 M/S EASTERN MEDIKIT LIMITED M/S MADAMSER & CO N - 22, GREATER KAILASH, PART - 1, FLATNO.E(G.F) SAGAR APARTMENTS, NEW DELHI - 48. 6, TILAK MARG, V/S NEW DELHI-110001. M/S ROMSONS SCIENTIFIC & SURGICAL V/S IND. PVT. LTD. M/S P.K.ARORA 63, INDL. ESTATE,NUNHAI, 110, ANAND VRINDAVAN AGRA SANJAY PALACE AGRA-02 (U.P.) TM-5 TIME BARRED 2 191277 1122329 M/S FILEX SYSTEMS PRIVATE LIMITED, M/S MANGLA REGISTRATION SERVICE, 3/27, ROOP NAGAR, DELHI - 110 007. 1961, KATRA SHAHN-SHAHI, V/S CHANDNI CHOWK, DELHI-110006. M/S WM. WRIGLEY JR. CO. V/S 410 NORTH MICHIGAN AVENUE, CHICAGO, M/S ANAND AND ANAND, 1L 60611, USA. B-41, NIZAMUDDIN EAST, NEW DELHI-11013. 3 170889 1086061 M/S BLAZE VELVET & COSMETICS (INDIA). SHRI AMIT SACHDEVA WZ - 19, NEAR M. C. D. SCHOOL, VILLAGE SHOP NO.2904/04, RUI MANDI, DASGHARA, NEW DELHI - 110 012. HANUMAN MARKET, SADAR BAZAR V/S DELHI-110006 M/S J.R.PRODUCTS V/S 55, VITHALJINA PATEL CHAWL, OPP PARK M/S M.P. MIRCHANDANI SIDE HOTEL, M.G.ROAD, SUKHERWADI, RAM MANSION, IST FLOOR, ROOM NO.4, BORIVALI (E) MUMBAI-400066. -
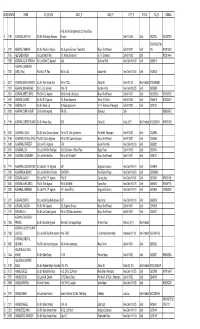
Main Voter List 08.01.2018.Pdf
Sl.NO ADM.NO NAME SO_DO_WO ADD1_R ADD2_R CITY_R STATE TEL_R MOBILE 61-B, Abul Fazal Apartments 22, Vasundhara 1 1150 ACHARJEE,AMITAVA S/o Shri Sudhamay Acharjee Enclave Delhi-110 096 Delhi 22620723 9312282751 22752142,22794 2 0181 ADHYARU,YASHANK S/o Shri Pravin K. Adhyaru 295, Supreme Enclave, Tower No.3, Mayur Vihar Phase-I Delhi-110 091 Delhi 745 9810813583 3 0155 AELTEMESH REIN S/o Late Shri M. Rein 107, Natraj Apartments 67, I.P. Extension Delhi-110 092 Delhi 9810214464 4 1298 AGARWAL,ALOK KRISHNA S/o Late Shri K.C. Agarwal A-56, Gulmohar Park New Delhi-110 049 Delhi 26851313 AGARWAL,DARSHANA 5 1337 (MRS.) (Faizi) W/o Shri O.P. Faizi Flat No. 258, Kailash Hills New Delhi-110 065 Delhi 51621300 6 0317 AGARWAL,MAM CHANDRA S/o Shri Ram Sharan Das Flat No.1133, Sector-29, Noida-201 301 Uttar Pradesh 0120-2453952 7 1427 AGARWAL,MOHAN BABU S/o Dr. C.B. Agarwal H.No. 78, Sukhdev Vihar New Delhi-110 025 Delhi 26919586 8 1021 AGARWAL,NEETA (MRS.) W/o Shri K.C. Agarwal B-608, Anand Lok Society Mayur Vihar Phase-I Delhi-110 091 Delhi 9312059240 9810139122 9 0687 AGARWAL,RAJEEV S/o Shri R.C. Agarwal 244, Bharat Apartment Sector-13, Rohini Delhi-110 085 Delhi 27554674 9810028877 11 1400 AGARWAL,S.K. S/o Shri Kishan Lal 78, Kirpal Apartments 44, I.P. Extension, Patparganj Delhi-110 092 Delhi 22721132 12 0933 AGARWAL,SUNIL KUMAR S/o Murlidhar Agarwal WB-106, Shakarpur, Delhi 9868036752 13 1199 AGARWAL,SURESH KUMAR S/o Shri Narain Dass B-28, Sector-53 Noida, (UP) Uttar Pradesh0120-2583477 9818791243 15 0242 AGGARWAL,ARUN S/o Shri Uma Shankar Agarwal Flat No.26, Trilok Apartments Plot No.85, Patparganj Delhi-110 092 Delhi 22433988 16 0194 AGGARWAL,MRIDUL (MRS.) W/o Shri Rajesh Aggarwal Flat No.214, Supreme Enclave Mayur Vihar Phase-I, Delhi-110 091 Delhi 22795565 17 0484 AGGARWAL,PRADEEP S/o Late R.P. -

611B Bus Time Schedule & Line Route
611B bus time schedule & line map 611B Mayur Vihar Phase III Terminal / Paper Market View In Website Mode The 611B bus line (Mayur Vihar Phase III Terminal / Paper Market) has 2 routes. For regular weekdays, their operation hours are: (1) Mayur Vihar Phase III Terminal / Paper Market: 7:40 AM - 9:50 PM (2) Rk Puram Sec-1: 6:10 AM - 9:40 PM Use the Moovit App to ƒnd the closest 611B bus station near you and ƒnd out when is the next 611B bus arriving. Direction: Mayur Vihar Phase III Terminal / Paper 611B bus Time Schedule Market Mayur Vihar Phase III Terminal / Paper Market Route 48 stops Timetable: VIEW LINE SCHEDULE Sunday 7:40 AM - 9:50 PM Monday 7:40 AM - 9:50 PM R K Puram Sec-1 Tuesday 7:40 AM - 9:50 PM R K Puram Sec-4 Wednesday 7:40 AM - 9:50 PM R K Puram Sec-3 Thursday 7:40 AM - 9:50 PM Rk Puram Sec-3 Friday 7:40 AM - 9:50 PM Venkateshwara Marg, New Delhi Saturday 7:40 AM - 9:50 PM R K Puram Sec-2 Mohammadpur Village B-1/12 Safdarjung Enclave, New Delhi 611B bus Info Safdarjung Enclave Direction: Mayur Vihar Phase III Terminal / Paper Market Green Field School Stops: 48 Trip Duration: 61 min Kamal Cinema Line Summary: R K Puram Sec-1, R K Puram Sec-4, R K Puram Sec-3, Rk Puram Sec-3, R K Puram Sec-2, Mohammadpur Village, Safdarjung Enclave, Green Raj Nagar Field School, Kamal Cinema, Raj Nagar, SJ Hospital, Aiims Ring Road, South Extension, South Extension, SJ Hospital Andrews Ganj, Gupta Market, Lajpat Nagar 2 Ring Road, Lajpat Nagar 1 Ring Road, PG DAV College / Aiims Ring Road Sri Niwaspuri, Nehru Nagar, Maharani Bagh / Ashram, -

ML-70 Bus Time Schedule & Line Route
ML-70 bus time schedule & line map ML-70 Dhaula Kuan Metro Station - Ambedkar Nagar View In Website Mode Sector-5 The ML-70 bus line (Dhaula Kuan Metro Station - Ambedkar Nagar Sector-5) has 2 routes. For regular weekdays, their operation hours are: (1) Ambedkar Nagar Sector-5: 6:00 AM - 10:00 PM (2) Dhaula Kuan Metro Station: 6:00 AM - 10:00 PM Use the Moovit App to ƒnd the closest ML-70 bus station near you and ƒnd out when is the next ML-70 bus arriving. Direction: Ambedkar Nagar Sector-5 ML-70 bus Time Schedule 47 stops Ambedkar Nagar Sector-5 Route Timetable: VIEW LINE SCHEDULE Sunday 6:00 AM - 10:00 PM Monday 6:00 AM - 10:00 PM Dhaula Kuan Metro Station Tuesday 6:00 AM - 10:00 PM Dhaula Kuan Wednesday 6:00 AM - 10:00 PM Satya Niketan Dhaula Kuan Thursday 6:00 AM - 10:00 PM Moti Bagh Gurudwara Nanakpura Friday 6:00 AM - 10:00 PM South Moti Bagh (Ring Road) Saturday 6:00 AM - 10:00 PM North Moti Bagh Aradhana Enclave ML-70 bus Info Rk Puram Sec-13 Direction: Ambedkar Nagar Sector-5 Stops: 47 Mahatma Gandhi Marg, New Delhi Trip Duration: 47 min Line Summary: Dhaula Kuan Metro Station, Dhaula Hyatt Hotel Kuan, Satya Niketan Dhaula Kuan, Moti Bagh Gurudwara Nanakpura, South Moti Bagh (Ring Bhikaji Cama Place Road), North Moti Bagh, Aradhana Enclave, Rk Puram Sec-13, Hyatt Hotel, Bhikaji Cama Place, Mohammadpur Village Mohammadpur Village, R K Puram Sec-2, Rk Khanna B-1/12 Safdarjung Enclave, New Delhi Tennis Stadium, IIT Hostel, Ber Sarai, Ber Sarai, School Of Physical Science, F.A.I., Sanskrit R K Puram Sec-2 Vidyapeeth, Katwaria Sarai, -

MASTER PLAN for DELHI 2021.Pmd
MASTER PLAN FOR DELHI WITH THE PERSPECTIVE FOR THE YEAR 2021 IT IS A COPIED ONE FOREWORD The National Capital Territory (NCD) of Delhi has been withnessing unprecedented growth of popu- lation ever since Independence (from 17.4 lakhs in 1951 to 138.5 lakhs in 2001). With the area remaining static (at 1487 sq. km), the need for systematic planning has been assuming increasing im- portance and urgency. After some interim preparatory steps, the Delhi Development Authority (DDA) came into existence after enactment of the Delhi Development Act, 1957. The first comprehensive Master Plan for Delhi (MPD) was brought into force on the Ist. September 1962, with a perspective of 20 years, i.e., up to 1982. The 2nd. Master Plan (Perspective 2001) due before 1982, came out only in 1990. This plan (MPD-2021) that ought to have been in public domain before 2001, has come now in 2007. Planning is a dynamic process that demands continuous fine-tuning to suit emerging needs and attitudes; to remedy what is hurting the people, to draw lessons from the past, to anticipate the needs and aspirations of the people in times to come. In the democratic system that ‘We the People of india’ have adopted for ourselves, it becomes the duty of we the citizens of Delhi to world, one of which all the Indians should feel proud. For this to happen it is imperative that the Master Plan is perceived as People’s Document and not a mere Sarkari Plan. This requires that the people at larg read the MPD, grasp its concept and implications, critically examine if its provisions are leading Delhi in the right direction and most importantly, liberally offer their views and suggest innovations and improvements.