Atlantic Needlefish)
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Belonidae Bonaparte 1832 Needlefishes
ISSN 1545-150X California Academy of Sciences A N N O T A T E D C H E C K L I S T S O F F I S H E S Number 16 September 2003 Family Belonidae Bonaparte 1832 needlefishes By Bruce B. Collette National Marine Fisheries Service Systematics Laboratory National Museum of Natural History, Washington, DC 20560–0153, U.S.A. email: [email protected] Needlefishes are a relatively small family of beloniform fishes (Rosen and Parenti 1981 [ref. 5538], Collette et al. 1984 [ref. 11422]) that differ from other members of the order in having both the upper and the lower jaws extended into long beaks filled with sharp teeth (except in the neotenic Belonion), the third pair of upper pharyngeal bones separate, scales on the body relatively small, and no finlets following the dorsal and anal fins. The nostrils lie in a pit anterior to the eyes. There are no spines in the fins. The dorsal fin, with 11–43 rays, and anal fin, with 12–39 rays, are posterior in position; the pelvic fins, with 6 soft rays, are located in an abdominal position; and the pectoral fins are short, with 5–15 rays. The lateral line runs down from the pectoral fin origin and then along the ventral margin of the body. The scales are small, cycloid, and easily detached. Precaudal vertebrae number 33–65, caudal vertebrae 19–41, and total verte- brae 52–97. Some freshwater needlefishes reach only 6 or 7 cm (2.5 or 2.75 in) in total length while some marine species may attain 2 m (6.5 ft). -

Tennessee Fish Species
The Angler’s Guide To TennesseeIncluding Aquatic Nuisance SpeciesFish Published by the Tennessee Wildlife Resources Agency Cover photograph Paul Shaw Graphics Designer Raleigh Holtam Thanks to the TWRA Fisheries Staff for their review and contributions to this publication. Special thanks to those that provided pictures for use in this publication. Partial funding of this publication was provided by a grant from the United States Fish & Wildlife Service through the Aquatic Nuisance Species Task Force. Tennessee Wildlife Resources Agency Authorization No. 328898, 58,500 copies, January, 2012. This public document was promulgated at a cost of $.42 per copy. Equal opportunity to participate in and benefit from programs of the Tennessee Wildlife Resources Agency is available to all persons without regard to their race, color, national origin, sex, age, dis- ability, or military service. TWRA is also an equal opportunity/equal access employer. Questions should be directed to TWRA, Human Resources Office, P.O. Box 40747, Nashville, TN 37204, (615) 781-6594 (TDD 781-6691), or to the U.S. Fish and Wildlife Service, Office for Human Resources, 4401 N. Fairfax Dr., Arlington, VA 22203. Contents Introduction ...............................................................................1 About Fish ..................................................................................2 Black Bass ...................................................................................3 Crappie ........................................................................................7 -

© Iccat, 2007
A5 By-catch Species APPENDIX 5: BY-CATCH SPECIES A.5 By-catch species By-catch is the unintentional/incidental capture of non-target species during fishing operations. Different types of fisheries have different types and levels of by-catch, depending on the gear used, the time, area and depth fished, etc. Article IV of the Convention states: "the Commission shall be responsible for the study of the population of tuna and tuna-like fishes (the Scombriformes with the exception of Trichiuridae and Gempylidae and the genus Scomber) and such other species of fishes exploited in tuna fishing in the Convention area as are not under investigation by another international fishery organization". The following is a list of by-catch species recorded as being ever caught by any major tuna fishery in the Atlantic/Mediterranean. Note that the lists are qualitative and are not indicative of quantity or mortality. Thus, the presence of a species in the lists does not imply that it is caught in significant quantities, or that individuals that are caught necessarily die. Skates and rays Scientific names Common name Code LL GILL PS BB HARP TRAP OTHER Dasyatis centroura Roughtail stingray RDC X Dasyatis violacea Pelagic stingray PLS X X X X Manta birostris Manta ray RMB X X X Mobula hypostoma RMH X Mobula lucasana X Mobula mobular Devil ray RMM X X X X X Myliobatis aquila Common eagle ray MYL X X Pteuromylaeus bovinus Bull ray MPO X X Raja fullonica Shagreen ray RJF X Raja straeleni Spotted skate RFL X Rhinoptera spp Cownose ray X Torpedo nobiliana Torpedo -

Fishes of Terengganu East Coast of Malay Peninsula, Malaysia Ii Iii
i Fishes of Terengganu East coast of Malay Peninsula, Malaysia ii iii Edited by Mizuki Matsunuma, Hiroyuki Motomura, Keiichi Matsuura, Noor Azhar M. Shazili and Mohd Azmi Ambak Photographed by Masatoshi Meguro and Mizuki Matsunuma iv Copy Right © 2011 by the National Museum of Nature and Science, Universiti Malaysia Terengganu and Kagoshima University Museum All rights reserved. No part of this publication may be reproduced or transmitted in any form or by any means without prior written permission from the publisher. Copyrights of the specimen photographs are held by the Kagoshima Uni- versity Museum. For bibliographic purposes this book should be cited as follows: Matsunuma, M., H. Motomura, K. Matsuura, N. A. M. Shazili and M. A. Ambak (eds.). 2011 (Nov.). Fishes of Terengganu – east coast of Malay Peninsula, Malaysia. National Museum of Nature and Science, Universiti Malaysia Terengganu and Kagoshima University Museum, ix + 251 pages. ISBN 978-4-87803-036-9 Corresponding editor: Hiroyuki Motomura (e-mail: [email protected]) v Preface Tropical seas in Southeast Asian countries are well known for their rich fish diversity found in various environments such as beautiful coral reefs, mud flats, sandy beaches, mangroves, and estuaries around river mouths. The South China Sea is a major water body containing a large and diverse fish fauna. However, many areas of the South China Sea, particularly in Malaysia and Vietnam, have been poorly studied in terms of fish taxonomy and diversity. Local fish scientists and students have frequently faced difficulty when try- ing to identify fishes in their home countries. During the International Training Program of the Japan Society for Promotion of Science (ITP of JSPS), two graduate students of Kagoshima University, Mr. -

Updated Checklist of Marine Fishes (Chordata: Craniata) from Portugal and the Proposed Extension of the Portuguese Continental Shelf
European Journal of Taxonomy 73: 1-73 ISSN 2118-9773 http://dx.doi.org/10.5852/ejt.2014.73 www.europeanjournaloftaxonomy.eu 2014 · Carneiro M. et al. This work is licensed under a Creative Commons Attribution 3.0 License. Monograph urn:lsid:zoobank.org:pub:9A5F217D-8E7B-448A-9CAB-2CCC9CC6F857 Updated checklist of marine fishes (Chordata: Craniata) from Portugal and the proposed extension of the Portuguese continental shelf Miguel CARNEIRO1,5, Rogélia MARTINS2,6, Monica LANDI*,3,7 & Filipe O. COSTA4,8 1,2 DIV-RP (Modelling and Management Fishery Resources Division), Instituto Português do Mar e da Atmosfera, Av. Brasilia 1449-006 Lisboa, Portugal. E-mail: [email protected], [email protected] 3,4 CBMA (Centre of Molecular and Environmental Biology), Department of Biology, University of Minho, Campus de Gualtar, 4710-057 Braga, Portugal. E-mail: [email protected], [email protected] * corresponding author: [email protected] 5 urn:lsid:zoobank.org:author:90A98A50-327E-4648-9DCE-75709C7A2472 6 urn:lsid:zoobank.org:author:1EB6DE00-9E91-407C-B7C4-34F31F29FD88 7 urn:lsid:zoobank.org:author:6D3AC760-77F2-4CFA-B5C7-665CB07F4CEB 8 urn:lsid:zoobank.org:author:48E53CF3-71C8-403C-BECD-10B20B3C15B4 Abstract. The study of the Portuguese marine ichthyofauna has a long historical tradition, rooted back in the 18th Century. Here we present an annotated checklist of the marine fishes from Portuguese waters, including the area encompassed by the proposed extension of the Portuguese continental shelf and the Economic Exclusive Zone (EEZ). The list is based on historical literature records and taxon occurrence data obtained from natural history collections, together with new revisions and occurrences. -
*For More Information, Please See
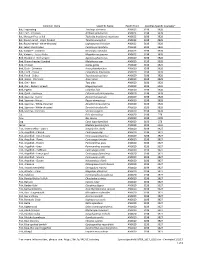
Common Name Scientific Name Health Point Specifies-Specific Course(s)* Bat, Frog-eating Trachops cirrhosus AN0023 3198 3928 Bat, Fruit - Jamaican Artibeus jamaicensis AN0023 3198 3928 Bat, Mexican Free-tailed Tadarida brasiliensis mexicana AN0023 3198 3928 Bat, Round-eared - stripe-headed Tonatia saurophila AN0023 3198 3928 Bat, Round-eared - white-throated Lophostoma silvicolum AN0023 3198 3928 Bat, Seba's short-tailed Carollia perspicillata AN0023 3198 3928 Bat, Vampire - Common Desmodus rotundus AN0023 3198 3928 Bat, Vampire - Lesser False Megaderma spasma AN0023 3198 3928 Bird, Blackbird - Red-winged Agelaius phoeniceus AN0020 3198 3928 Bird, Brown-headed Cowbird Molothurus ater AN0020 3198 3928 Bird, Chicken Gallus gallus AN0020 3198 3529 Bird, Duck - Domestic Anas platyrhynchos AN0020 3198 3928 Bird, Finch - House Carpodacus mexicanus AN0020 3198 3928 Bird, Finch - Zebra Taeniopygia guttata AN0020 3198 3928 Bird, Goose - Domestic Anser anser AN0020 3198 3928 Bird, Owl - Barn Tyto alba AN0020 3198 3928 Bird, Owl - Eastern Screech Megascops asio AN0020 3198 3928 Bird, Pigeon Columba livia AN0020 3198 3928 Bird, Quail - Japanese Coturnix coturnix japonica AN0020 3198 3928 Bird, Sparrow - Harris' Zonotrichia querula AN0020 3198 3928 Bird, Sparrow - House Passer domesticus AN0020 3198 3928 Bird, Sparrow - White-crowned Zonotrichia leucophrys AN0020 3198 3928 Bird, Sparrow - White-throated Zonotrichia albicollis AN0020 3198 3928 Bird, Starling - Common Sturnus vulgaris AN0020 3198 3928 Cat Felis domesticus AN0020 3198 279 Cow Bos taurus -
St. Joseph Bay Native Species List
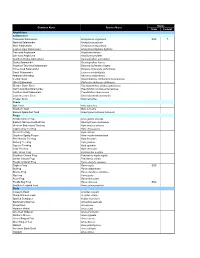
Status Common Name Species Name State Federal Amphibians Salamanders Flatwoods Salamander Ambystoma cingulatum SSC T Marbled Salamander Ambystoma opacum Mole Salamander Ambystoma talpoideum Eastern Tiger Salamander Ambystoma tigrinum tigrinum Two-toed Amphiuma Amphiuma means One-toed Amphiuma Amphiuma pholeter Southern Dusky Salamander Desmognathus auriculatus Dusky Salamander Desmognathus fuscus Southern Two-lined Salamander Eurycea bislineata cirrigera Three-lined Salamander Eurycea longicauda guttolineata Dwarf Salamander Eurycea quadridigitata Alabama Waterdog Necturus alabamensis Central Newt Notophthalmus viridescens louisianensis Slimy Salamander Plethodon glutinosus glutinosus Slender Dwarf Siren Pseudobranchus striatus spheniscus Gulf Coast Mud Salamander Pseudotriton montanus flavissimus Southern Red Salamander Pseudotriton ruber vioscai Eastern Lesser Siren Siren intermedia intermedia Greater Siren Siren lacertina Toads Oak Toad Bufo quercicus Southern Toad Bufo terrestris Eastern Spadefoot Toad Scaphiopus holbrooki holbrooki Frogs Florida Cricket Frog Acris gryllus dorsalis Eastern Narrow-mouthed Frog Gastrophryne carolinensis Western Bird-voiced Treefrog Hyla avivoca avivoca Cope's Gray Treefrog Hyla chrysoscelis Green Treefrog Hyla cinerea Southern Spring Peeper Hyla crucifer bartramiana Pine Woods Treefrog Hyla femoralis Barking Treefrog Hyla gratiosa Squirrel Treefrog Hyla squirella Gray Treefrog Hyla versicolor Little Grass Frog Limnaoedus ocularis Southern Chorus Frog Pseudacris nigrita nigrita Ornate Chorus Frog Pseudacris -

Extinction Rates in North American Freshwater Fishes, 1900–2010 Author(S): Noel M
Extinction Rates in North American Freshwater Fishes, 1900–2010 Author(s): Noel M. Burkhead Source: BioScience, 62(9):798-808. 2012. Published By: American Institute of Biological Sciences URL: http://www.bioone.org/doi/full/10.1525/bio.2012.62.9.5 BioOne (www.bioone.org) is a nonprofit, online aggregation of core research in the biological, ecological, and environmental sciences. BioOne provides a sustainable online platform for over 170 journals and books published by nonprofit societies, associations, museums, institutions, and presses. Your use of this PDF, the BioOne Web site, and all posted and associated content indicates your acceptance of BioOne’s Terms of Use, available at www.bioone.org/page/terms_of_use. Usage of BioOne content is strictly limited to personal, educational, and non-commercial use. Commercial inquiries or rights and permissions requests should be directed to the individual publisher as copyright holder. BioOne sees sustainable scholarly publishing as an inherently collaborative enterprise connecting authors, nonprofit publishers, academic institutions, research libraries, and research funders in the common goal of maximizing access to critical research. Articles Extinction Rates in North American Freshwater Fishes, 1900–2010 NOEL M. BURKHEAD Widespread evidence shows that the modern rates of extinction in many plants and animals exceed background rates in the fossil record. In the present article, I investigate this issue with regard to North American freshwater fishes. From 1898 to 2006, 57 taxa became extinct, and three distinct populations were extirpated from the continent. Since 1989, the numbers of extinct North American fishes have increased by 25%. From the end of the nineteenth century to the present, modern extinctions varied by decade but significantly increased after 1950 (post-1950s mean = 7.5 extinct taxa per decade). -

Tylosurus Acus Imperialis (Osteichthyes: Belonidae), a Fish New to the Adriatic Sea
Cah. Biol. Mar. (1995) 36 : 197-199 Tylosurus acus imperialis (Osteichthyes: Belonidae), a fish new to the Adriatic Sea G. BELLO Laboratorio Provinciale di Biologia Marina, Molo Pizzoli - 70123 Bari, Italy. Abstract: Two male and one female specimens of the agujon needlefish, Tylosurus acus imperialis, were collected off the south-western Adriatic coast. This finding extends the distribution of the species to the Adriatic Sea. The examination of the ripe female showed that T. acus imperialis is a multiple spawner and that its eggs have sticky filaments, as is typical of the family Belonidae. Résumé: Deux spécimens mâles et une femelle d'Aiguille voyeuse, Tylosurus acus imperialis, ont été récoltés au large de la côte sud-ouest de la mer Adriatique, ce qui étend la distribution de l'espèce à cette région. L'examen de l'ovaire de la femelle sexuellement mûre montre que cette espèce pond plusieurs fois et que ses œufs sont pourvus de filaments adhésifs, ce qui est typique de la famille des Belonidae. Keywords: Osteichthyes, Tylosurus acus imperialis, occurrence, Adriatic Sea, oocyte. Introduction (Bini, 1970; Parin, 1973; Collette & Parin, 1986; Bauchot, The needlefish family, Belonidae; is represented in the 1987). It has never b~en recorded in the Adriatic Sea (see Mediterrandm Sea by four species, namely Belone belone negative evidence in Soljan, 1975). (Linnaeus, 1761), Belone svetovidovi Collette & Parin, 1970, Tylosurus acus (Lacepède, 1803), and Tylosurus Materials and methods choram (Rüppell, 1837); the last is a Lessepsian migrant Two specimens of T. acus imperialis were captured along (Collette & Parin, 1986; Bauchot, 1987). the coast of Mola di Bari (Italy, South Adriatic Sea) on 23 According to the revision by Collette & Parin (1970), the May 1994. -

Changes in the Fish Community of a Western Caribbean Estuary After the Expansion of an Artificial Channel to the Sea
water Article Changes in the Fish Community of a Western Caribbean Estuary after the Expansion of an Artificial Channel to the Sea Juan J. Schmitter-Soto * and Roberto L. Herrera-Pavón El Colegio de la Frontera Sur, Av. Centenario km 5.5, Chetumal 77014, Quintana Roo, Mexico; [email protected] * Correspondence: [email protected]; Tel.: +52-983-835-0440 (ext. 4302) Received: 30 October 2019; Accepted: 2 December 2019; Published: 6 December 2019 Abstract: Increased connectivity between coastal lagoons and the sea is expected to entail a greater proportion of marine species in the former. Chetumal Bay, estuary of the Hondo river into the Caribbean, had a limited access to the sea until the opening of the Zaragoza Canal. We sought changes in the fish community from 1999–2001 (just after an expansion of the canal) to 2015–2018. The same fishing gear was used, in the same localities, during all seasons. Total fish abundance and mean local richness decreased, although total abundance increased in the polyhaline zone. Diversity was greater in the oligohaline zone in 1999–2001, and in the mesohaline zone in 2015–2018. Three guilds were absent in 2015–2018: Medium-sized herbivores, large piscivores, and medium-sized planktivores. Abundance of small benthivores decreased by decade; medium-sized piscivores and small planktivores became more abundant in 2015–2018 in the polyhaline zone. These changes may be due to the opening of the channel, but illegal fishing outside the bay may explain the decrease in juveniles of large piscivores, and erosion in the innermost part may be destroying important habitats. -

FISHES (C) Val Kells–November, 2019
VAL KELLS Marine Science Illustration 4257 Ballards Mill Road - Free Union - VA - 22940 www.valkellsillustration.com [email protected] STOCK ILLUSTRATION LIST FRESHWATER and SALTWATER FISHES (c) Val Kells–November, 2019 Eastern Atlantic and Gulf of Mexico: brackish and saltwater fishes Subject to change. New illustrations added weekly. Atlantic hagfish, Myxine glutinosa Sea lamprey, Petromyzon marinus Deepwater chimaera, Hydrolagus affinis Atlantic spearnose chimaera, Rhinochimaera atlantica Nurse shark, Ginglymostoma cirratum Whale shark, Rhincodon typus Sand tiger, Carcharias taurus Ragged-tooth shark, Odontaspis ferox Crocodile Shark, Pseudocarcharias kamoharai Thresher shark, Alopias vulpinus Bigeye thresher, Alopias superciliosus Basking shark, Cetorhinus maximus White shark, Carcharodon carcharias Shortfin mako, Isurus oxyrinchus Longfin mako, Isurus paucus Porbeagle, Lamna nasus Freckled Shark, Scyliorhinus haeckelii Marbled catshark, Galeus arae Chain dogfish, Scyliorhinus retifer Smooth dogfish, Mustelus canis Smalleye Smoothhound, Mustelus higmani Dwarf Smoothhound, Mustelus minicanis Florida smoothhound, Mustelus norrisi Gulf Smoothhound, Mustelus sinusmexicanus Blacknose shark, Carcharhinus acronotus Bignose shark, Carcharhinus altimus Narrowtooth Shark, Carcharhinus brachyurus Spinner shark, Carcharhinus brevipinna Silky shark, Carcharhinus faiformis Finetooth shark, Carcharhinus isodon Galapagos Shark, Carcharhinus galapagensis Bull shark, Carcharinus leucus Blacktip shark, Carcharhinus limbatus Oceanic whitetip shark, -

Fishes of the Lemon Bay Estuary and a Comparison of Fish Community Structure to Nearby Estuaries Along Florida’S Gulf Coast
Biological Sciences Fishes of the Lemon Bay estuary and a comparison of fish community structure to nearby estuaries along Florida’s Gulf coast Charles F. Idelberger(1), Philip W. Stevens(2), and Eric Weather(2) (1)Florida Fish and Wildlife Conservation Commission, Fish and Wildlife Research Institute, Charlotte Harbor Field Laboratory, 585 Prineville Street, Port Charlotte, Florida 33954 (2)Florida Fish and Wildlife Conservation Commission, Fish and Wildlife Research Institute, 100 Eighth Avenue Southeast, Saint Petersburg, Florida 33701 Abstract Lemon Bay is a narrow, shallow estuary in southwest Florida. Although its fish fauna has been studied intermittently since the 1880s, no detailed inventory has been available. We sampled fish and selected macroinvertebrates in the bay and lower portions of its tributaries from June 2009 through April 2010 using seines and trawls. One hundred three fish and six invertebrate taxa were collected. Pinfish Lagodon rhomboides, spot Leiostomus xanthurus, bay anchovy Anchoa mitchilli, mojarras Eucinostomus spp., silver perch Bairdiella chrysoura, and scaled sardine Harengula jaguana were among the most abundant species. To place our information into a broader ecological context, we compared the Lemon Bay fish assemblages with those of nearby estuaries. Multivariate analyses revealed that fish assemblages of Lemon and Sarasota bays differed from those of lower Charlotte Harbor and lower Tampa Bay at similarities of 68–75%, depending on collection gear. These differences were attributed to greater abundances of small-bodied fishes in Lemon and Sarasota bays than in the other much larger estuaries. Factors such as water circulation patterns, length of shoreline relative to area of open water, and proximity of Gulf passes to juvenile habitat may differ sufficiently between the small and large estuaries to affect fish assemblages.