The Potential for Cobalt in the UK MAY 2020
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Developing a Solvent Extraction Process for the Separation of Cobalt and Iron from Nickel Sulfate Solutions
Abstract DEVELOPING A SOLVENT EXTRACTION PROCESS FOR THE SEPARATION OF COBALT AND IRON FROM NICKEL SULFATE SOLUTIONS By Michiel Casparus Olivier Thesis presented in partial fulfilment of the requirements for the Degree of MASTER OF SCIENCE IN ENGINEERING (EXTRACTIVE METALLURGICAL ENGINEERING) In the faculty of Engineering at Stellenbosch University Supervised by Christie Dorfling December 2011 i Stellenbosch University http://scholar.sun.ac.za DeclarationAbstract DECLARATION By submitting this thesis electronically, I declare that the entirety of the work contained therein is my own, original work, that I am the sole author thereof (save to the extent explicitly otherwise stated), that reproduction and publication thereof by Stellenbosch University will not infringe any third party rights and that I have not previously in its entirety or in part submitted it for obtaining any qualification. …………………………… ………………. Signature Date Copyright © 2011 Stellenbosch University All rights reserved i Stellenbosch University http://scholar.sun.ac.za Abstract ABSTRACT Crude NiSO4 solutions are often produced as a product of Sherrit based matte leach processes leading to iron and cobalt contaminated solutions of NiSO4. To upgrade the quality of these solutions for either, the production of NiSO4 crystals or cathode/precipitated nickel, the iron and cobalt must be removed. Conventional processes use either pure or saponified Cyanex 272 in solvent extraction to extract iron and cobalt from pregnant nickel leach solutions. These processes require the addition of an alkali like NaOH to neutralise the protons being exchanged for the different metal species since extraction is a strong function of pH. Hence, while removing iron and cobalt from the solution, sodium is added instead. -

LOW TEMPERATURE HYDROTHERMAL COPPER, NICKEL, and COBALT ARSENIDE and SULFIDE ORE FORMATION Nicholas Allin
Montana Tech Library Digital Commons @ Montana Tech Graduate Theses & Non-Theses Student Scholarship Spring 2019 EXPERIMENTAL INVESTIGATION OF THE THERMOCHEMICAL REDUCTION OF ARSENITE AND SULFATE: LOW TEMPERATURE HYDROTHERMAL COPPER, NICKEL, AND COBALT ARSENIDE AND SULFIDE ORE FORMATION Nicholas Allin Follow this and additional works at: https://digitalcommons.mtech.edu/grad_rsch Part of the Geotechnical Engineering Commons EXPERIMENTAL INVESTIGATION OF THE THERMOCHEMICAL REDUCTION OF ARSENITE AND SULFATE: LOW TEMPERATURE HYDROTHERMAL COPPER, NICKEL, AND COBALT ARSENIDE AND SULFIDE ORE FORMATION by Nicholas C. Allin A thesis submitted in partial fulfillment of the requirements for the degree of Masters in Geoscience: Geology Option Montana Technological University 2019 ii Abstract Experiments were conducted to determine the relative rates of reduction of aqueous sulfate and aqueous arsenite (As(OH)3,aq) using foils of copper, nickel, or cobalt as the reductant, at temperatures of 150ºC to 300ºC. At the highest temperature of 300°C, very limited sulfate reduction was observed with cobalt foil, but sulfate was reduced to sulfide by copper foil (precipitation of Cu2S (chalcocite)) and partly reduced by nickel foil (precipitation of NiS2 (vaesite) + NiSO4·xH2O). In the 300ºC arsenite reduction experiments, Cu3As (domeykite), Ni5As2, or CoAs (langisite) formed. In experiments where both sulfate and arsenite were present, some produced minerals were sulfarsenides, which contained both sulfide and arsenide, i.e. cobaltite (CoAsS). These experiments also produced large (~10 µm along longest axis) euhedral crystals of metal-sulfide that were either imbedded or grown upon a matrix of fine-grained metal-arsenides, or, in the case of cobalt, metal-sulfarsenide. Some experimental results did not show clear mineral formation, but instead demonstrated metal-arsenic alloying at the foil edges. -

Washington State Minerals Checklist
Division of Geology and Earth Resources MS 47007; Olympia, WA 98504-7007 Washington State 360-902-1450; 360-902-1785 fax E-mail: [email protected] Website: http://www.dnr.wa.gov/geology Minerals Checklist Note: Mineral names in parentheses are the preferred species names. Compiled by Raymond Lasmanis o Acanthite o Arsenopalladinite o Bustamite o Clinohumite o Enstatite o Harmotome o Actinolite o Arsenopyrite o Bytownite o Clinoptilolite o Epidesmine (Stilbite) o Hastingsite o Adularia o Arsenosulvanite (Plagioclase) o Clinozoisite o Epidote o Hausmannite (Orthoclase) o Arsenpolybasite o Cairngorm (Quartz) o Cobaltite o Epistilbite o Hedenbergite o Aegirine o Astrophyllite o Calamine o Cochromite o Epsomite o Hedleyite o Aenigmatite o Atacamite (Hemimorphite) o Coffinite o Erionite o Hematite o Aeschynite o Atokite o Calaverite o Columbite o Erythrite o Hemimorphite o Agardite-Y o Augite o Calciohilairite (Ferrocolumbite) o Euchroite o Hercynite o Agate (Quartz) o Aurostibite o Calcite, see also o Conichalcite o Euxenite o Hessite o Aguilarite o Austinite Manganocalcite o Connellite o Euxenite-Y o Heulandite o Aktashite o Onyx o Copiapite o o Autunite o Fairchildite Hexahydrite o Alabandite o Caledonite o Copper o o Awaruite o Famatinite Hibschite o Albite o Cancrinite o Copper-zinc o o Axinite group o Fayalite Hillebrandite o Algodonite o Carnelian (Quartz) o Coquandite o o Azurite o Feldspar group Hisingerite o Allanite o Cassiterite o Cordierite o o Barite o Ferberite Hongshiite o Allanite-Ce o Catapleiite o Corrensite o o Bastnäsite -

Rediscovery of the Elements — a Historical Sketch of the Discoveries
REDISCOVERY OF THE ELEMENTS — A HISTORICAL SKETCH OF THE DISCOVERIES TABLE OF CONTENTS incantations. The ancient Greeks were the first to Introduction ........................1 address the question of what these principles 1. The Ancients .....................3 might be. Water was the obvious basic 2. The Alchemists ...................9 essence, and Aristotle expanded the Greek 3. The Miners ......................14 philosophy to encompass a obscure mixture of 4. Lavoisier and Phlogiston ...........23 four elements — fire, earth, water, and air — 5. Halogens from Salts ...............30 as being responsible for the makeup of all 6. Humphry Davy and the Voltaic Pile ..35 materials of the earth. As late as 1777, scien- 7. Using Davy's Metals ..............41 tific texts embraced these four elements, even 8. Platinum and the Noble Metals ......46 though a over-whelming body of evidence 9. The Periodic Table ................52 pointed out many contradictions. It was taking 10. The Bunsen Burner Shows its Colors 57 thousands of years for mankind to evolve his 11. The Rare Earths .................61 thinking from Principles — which were 12. The Inert Gases .................68 ethereal notions describing the perceptions of 13. The Radioactive Elements .........73 this material world — to Elements — real, 14. Moseley and Atomic Numbers .....81 concrete basic stuff of this universe. 15. The Artificial Elements ...........85 The alchemists, who devoted untold Epilogue ..........................94 grueling hours to transmute metals into gold, Figs. 1-3. Mendeleev's Periodic Tables 95-97 believed that in addition to the four Aristo- Fig. 4. Brauner's 1902 Periodic Table ...98 telian elements, two principles gave rise to all Fig. 5. Periodic Table, 1925 ...........99 natural substances: mercury and sulfur. -
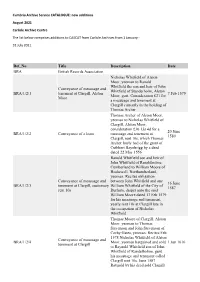
New Additions to CASCAT from Carlisle Archives
Cumbria Archive Service CATALOGUE: new additions August 2021 Carlisle Archive Centre The list below comprises additions to CASCAT from Carlisle Archives from 1 January - 31 July 2021. Ref_No Title Description Date BRA British Records Association Nicholas Whitfield of Alston Moor, yeoman to Ranald Whitfield the son and heir of John Conveyance of messuage and Whitfield of Standerholm, Alston BRA/1/2/1 tenement at Clargill, Alston 7 Feb 1579 Moor, gent. Consideration £21 for Moor a messuage and tenement at Clargill currently in the holding of Thomas Archer Thomas Archer of Alston Moor, yeoman to Nicholas Whitfield of Clargill, Alston Moor, consideration £36 13s 4d for a 20 June BRA/1/2/2 Conveyance of a lease messuage and tenement at 1580 Clargill, rent 10s, which Thomas Archer lately had of the grant of Cuthbert Baynbrigg by a deed dated 22 May 1556 Ranold Whitfield son and heir of John Whitfield of Ranaldholme, Cumberland to William Moore of Heshewell, Northumberland, yeoman. Recites obligation Conveyance of messuage and between John Whitfield and one 16 June BRA/1/2/3 tenement at Clargill, customary William Whitfield of the City of 1587 rent 10s Durham, draper unto the said William Moore dated 13 Feb 1579 for his messuage and tenement, yearly rent 10s at Clargill late in the occupation of Nicholas Whitfield Thomas Moore of Clargill, Alston Moor, yeoman to Thomas Stevenson and John Stevenson of Corby Gates, yeoman. Recites Feb 1578 Nicholas Whitfield of Alston Conveyance of messuage and BRA/1/2/4 Moor, yeoman bargained and sold 1 Jun 1616 tenement at Clargill to Raynold Whitfield son of John Whitfield of Randelholme, gent. -

Sudibyo S, Et Al. Optimization of Electrometal-Elektrowinning Cobalt Process from the Slag Copyright© Sudibyo S, Et Al
Physical Science & Biophysics Journal MEDWIN PUBLISHERS ISSN: 2641-9165 Committed to Create Value for Researchers Optimization of Electrometal-Elektrowinning Cobalt Process from the Slag of Nickel Pig Iron (NPI) Hermida L1, Sudibyo S2*, Supriyatna YI2, Herlina U2, Handoko AS2, Prasetyo E2, Almutaqii M2 and Reswari PA1 Research Article Volume 4 Issue 2 1Department of Chemical Engineering, Engineering Faculty, Lampung University, Indonesia Received Date: October 09, 2020 2Research Unit for Mineral Technology, Indonesian Institute of Sciences (LIPI), Indonesia Published Date: October 27, 2020 *Corresponding author: Sudibyo Sudibyo, Research Unit for Mineral Technology, Indonesian Institute of Sciences (LIPI), Ir. Sutami street Km. 15, Tanjung Bintang, South Lampung district, Lampung Province, Indonesia, Tel: +6285881020870; Email: [email protected] Abstract Slag from the manufacturing of nickel pig iron (NPI) from laterite soil is still containing 823.7 ppm of cobalt. In this research, the separation process is carried out from slag NPI by using the Response Surface Method (RSM). This method is to determine the optimum conditional process of Electrometal Electrowinning (EMEW) and get an equation model to see the correlation isbetween leach the a variable slag using and acetic know acid,the most and significantthen extracted interfactor in two interaction.steps by versatic This research acid 10 andwas thenconducted with cyanex using three 272. parameters,The organic phaseconsists from of duration this extraction of operation, then stripped potential using voltage, 6 M and sulphuric variable acid of boric so obtained acid. The aqueous first step phase in electro-metal at pH 5.5 with electrowinning the highest cobalt content. The best condition of electro-metal electrowinning is obtained at 4.5 V, 2 hours, and 0.5 M of boric acid with 45.8273% of cobalt recovery. -

Polymetallic Mineralization in Ediacaran Sediments in the Żarki-Kotowice Area, Poland
MINERALOGIA, 43, No 3-4: 199-212 (2012) DOI: 10.2478/v10002-012-0008-0 www.Mineralogia.pl MINERALOGICAL SOCIETY OF POLAND POLSKIE TOWARZYSTWO MINERALOGICZNE __________________________________________________________________________________________________________________________ Original paper Polymetallic mineralization in Ediacaran sediments in the Żarki-Kotowice area, Poland Łukasz KARWOWSKI1*, Marek MARKOWIAK2 1University of Silesia, Faculty of Earth Sciences, ul. Będzińska 60, 41-200 Sosnowiec, Poland; e-mail: [email protected] 2Polish Geological Institute - Research and Development Unit, Upper Silesian Branch, ul. Królowej Jadwigi 1, 41-200 Sosnowiec, Poland; e-mail: [email protected] * Corresponding author Received: September 5, 2012 Received in revised form: February 20, 2013 Accepted: March 17, 2013 Available online: March 30, 2013 Abstract. In one small mineral vein in core from borehole 144-Ż in the Żarki-Kotowice area, almost all of the ore minerals known from related deposits in the vicinity occur. Some of the minerals in the vein described in this paper, namely, nickeline, hessite, native silver and minerals of the cobaltite-gersdorffite group, have not previously been reported from elsewhere in the Kraków-Lubliniec tectonic zone. The identified minerals are chalcopyrite, pyrite, marcasite, sphalerite, Co-rich pyrite, tennantite, tetrahedrite, bornite, galena, magnetite, hematite, cassiterite, pyrrhotite, wolframite (ferberite), scheelite, molybdenite, nickeline, minerals of the cobaltite- gersdorffite group, carrollite, hessite and native silver. Moreover, native bismuth, bismuthinite, a Cu- and Ag-rich sulfosalt of Bi (cuprobismutite) and Ni-rich pyrite also occur in the vein. We suggest that, the ore mineralization from the borehole probably reflects post-magmatic hydrothermal activity related to an unseen granitic intrusion located under the Mesozoic sediments in the Żarki-Pilica area. -
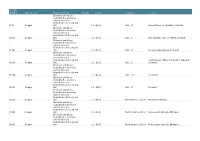
BRSUG Number Mineral Name Hey Index Group Hey No
BRSUG Number Mineral name Hey Index Group Hey No. Chem. Country Locality Elements and Alloys (including the arsenides, antimonides and bismuthides of Cu, Ag and B-37 Copper Au) 1.1 4[Cu] U.K., 17 Basset Mines, nr. Redruth, Cornwall Elements and Alloys (including the arsenides, antimonides and bismuthides of Cu, Ag and B-151 Copper Au) 1.1 4[Cu] U.K., 17 Phoenix mine, Cheese Wring, Cornwall Elements and Alloys (including the arsenides, antimonides and bismuthides of Cu, Ag and B-280 Copper Au) 1.1 4[Cu] U.K., 17 County Bridge Quarry, Cornwall Elements and Alloys (including the arsenides, antimonides and bismuthides of Cu, Ag and South Caradon Mine, 4 miles N of Liskeard, B-319 Copper Au) 1.1 4[Cu] U.K., 17 Cornwall Elements and Alloys (including the arsenides, antimonides and bismuthides of Cu, Ag and B-394 Copper Au) 1.1 4[Cu] U.K., 17 ? Cornwall? Elements and Alloys (including the arsenides, antimonides and bismuthides of Cu, Ag and B-395 Copper Au) 1.1 4[Cu] U.K., 17 Cornwall Elements and Alloys (including the arsenides, antimonides and bismuthides of Cu, Ag and B-539 Copper Au) 1.1 4[Cu] North America, U.S.A Houghton, Michigan Elements and Alloys (including the arsenides, antimonides and bismuthides of Cu, Ag and B-540 Copper Au) 1.1 4[Cu] North America, U.S.A Keweenaw Peninsula, Michigan, Elements and Alloys (including the arsenides, antimonides and bismuthides of Cu, Ag and B-541 Copper Au) 1.1 4[Cu] North America, U.S.A Keweenaw Peninsula, Michigan, Elements and Alloys (including the arsenides, antimonides and bismuthides of Cu, -

Geochemistry, Mineralogy and Microbiology of Cobalt in Mining-Affected Environments
minerals Article Geochemistry, Mineralogy and Microbiology of Cobalt in Mining-Affected Environments Gabriel Ziwa 1,2,*, Rich Crane 1,2 and Karen A. Hudson-Edwards 1,2 1 Environment and Sustainability Institute, University of Exeter, Penryn TR10 9FE, UK; [email protected] (R.C.); [email protected] (K.A.H.-E.) 2 Camborne School of Mines, University of Exeter, Penryn TR10 9FE, UK * Correspondence: [email protected] Abstract: Cobalt is recognised by the European Commission as a “Critical Raw Material” due to its irreplaceable functionality in many types of modern technology, combined with its current high-risk status associated with its supply. Despite such importance, there remain major knowledge gaps with regard to the geochemistry, mineralogy, and microbiology of cobalt-bearing environments, particu- larly those associated with ore deposits and subsequent mining operations. In such environments, high concentrations of Co (up to 34,400 mg/L in mine water, 14,165 mg/kg in tailings, 21,134 mg/kg in soils, and 18,434 mg/kg in stream sediments) have been documented. Co is contained in ore and mine waste in a wide variety of primary (e.g., cobaltite, carrolite, and erythrite) and secondary (e.g., erythrite, heterogenite) minerals. When exposed to low pH conditions, a number of such minerals are 2+ known to undergo dissolution, typically forming Co (aq). At circumneutral pH, such aqueous Co can then become immobilised by co-precipitation and/or sorption onto Fe and Mn(oxyhydr)oxides. This paper brings together contemporary knowledge on such Co cycling across different mining environments. -

Cobalt Mineral Ecology
American Mineralogist, Volume 102, pages 108–116, 2017 Cobalt mineral ecology ROBERT M. HAZEN1,*, GRETHE HYSTAD2, JOSHUA J. GOLDEN3, DANIEL R. HUMMER1, CHAO LIU1, ROBERT T. DOWNS3, SHAUNNA M. MORRISON3, JOLYON RALPH4, AND EDWARD S. GREW5 1Geophysical Laboratory, Carnegie Institution, 5251 Broad Branch Road NW, Washington, D.C. 20015, U.S.A. 2Department of Mathematics, Computer Science, and Statistics, Purdue University Northwest, Hammond, Indiana 46323, U.S.A. 3Department of Geosciences, University of Arizona, 1040 East 4th Street, Tucson, Arizona 85721-0077, U.S.A. 4Mindat.org, 128 Mullards Close, Mitcham, Surrey CR4 4FD, U.K. 5School of Earth and Climate Sciences, University of Maine, Orono, Maine 04469, U.S.A. ABSTRACT Minerals containing cobalt as an essential element display systematic trends in their diversity and distribution. We employ data for 66 approved Co mineral species (as tabulated by the official mineral list of the International Mineralogical Association, http://rruff.info/ima, as of 1 March 2016), represent- ing 3554 mineral species-locality pairs (www.mindat.org and other sources, as of 1 March 2016). We find that cobalt-containing mineral species, for which 20% are known at only one locality and more than half are known from five or fewer localities, conform to a Large Number of Rare Events (LNRE) distribution. Our model predicts that at least 81 Co minerals exist in Earth’s crust today, indicating that at least 15 species have yet to be discovered—a minimum estimate because it assumes that new minerals will be found only using the same methods as in the past. Numerous additional cobalt miner- als likely await discovery using micro-analytical methods. -

Development of the New Method for Oxidized Nickel Ore Processing
NIOKR-2018 Theoretical and practical conference with international participation and School KnE Materials Science for young scientists «FERROALLOYS: Development prospects of metallurgy and machine building based on completed Research and Development» Volume 2019 Conference Paper Development of the New Method for Oxidized Nickel Ore Processing Boris Dmitrievich Khalezov, Oleg Vadimovich Zayakin, Alexey Sergeevich Gavrilov, and Vladimir Ivanovich Zhuchkov IMET UB RAS, Ekaterinburg, Russia Abstract In modern conditions of sharp fluctuations in nickel prices on world markets, the problem of profitability of processing Russian poor oxidized nickel ores (ONO) has arisen. As an alternative to the previously existing in Russia, a sulphidation-reducing smelting on a matte, a hydro-pyrometallurgical method has been proposed for the preparation of complex nickel-, chromium-, manganese-containing ferroalloys. At the first stage, hydrolytic precipitation (with sodium hydroxide) is considered as a method of processing production solutions from heap leaching of ONO. It is established that Corresponding Author: at pH = 5.5, Al is completely removed in the precipitate as hydroxide. After washing Oleg Vadimovich Zayakin from impurities and calcining, a concentrate containing 50 wt.% Al was obtained. At the [email protected] second stage, at pH = 9.5, more than 99% of nickel and cobalt oxides, as well as 92% Received: 5 February 2019 MnO and 46% MgO, are precipitated. Obtained after washing and calcination at 700∘C Accepted: 6 March 2019 oxide concentrate contains, by weight. %: 67 NiO; 3 CoO; 20 MnO; 9 MgO; 0,2 S. At the Published: 17 March 2019 third stage, a pyrometallurgical method for smelting complex ferroalloys of the Fe-Ni-Cr- Publishing services provided by Mn-Si system using refractory ferrosilicochrome as a reducing agent is proposed for the Knowledge E processing of nickel-containing concentrate. -

Study on the Extraction and Separation of Zinc, Cobalt, and Nickel Using Ionquest 801, Cyanex 272, and Their Mixtures
metals Article Study on the Extraction and Separation of Zinc, Cobalt, and Nickel Using Ionquest 801, Cyanex 272, and Their Mixtures Wensen Liu 1,2,3, Jian Zhang 3,4,5, Zhenya Xu 6, Jie Liang 1,* and Zhaowu Zhu 2,3,4,* 1 School of Chemical and Environmental Engineering, China University of Mining and Technology (Beijing), Beijing 100083, China; [email protected] 2 Innovation Academy for Green Manufacture, Chinese Academy of Sciences, Beijing 100190, China 3 National Engineering Laboratory for Hydrometallurgical Cleaner Production Technology, Beijing 100190, China; [email protected] 4 Key Laboratory of Green Process and Engineering, Institute of Process Engineering, Chinese Academy of Sciences, Beijing 100190, China 5 School of Chemistry and Chemical Engineering, University of Chinese Academy of Sciences, Beijing 100049, China 6 The College of Chemical Engineering, Beijing University of Chemical Technology, Beijing 100028, China; [email protected] * Correspondence: [email protected] (J.L.); [email protected] (Z.Z.) Abstract: Both Cyanex 272 (bis (2,4,4-trimethylpentyl) phosphinic acid) and Ionquest 801 (2-ethylhexyl phosphonic acid mono-2-ethylhexyl ester) are commonly used for metal extraction and separation, particularly for zinc, cobalt, and nickel, which are often found together in processing solutions. Detailed metal extractions of zinc, cobalt, and nickel were studied in this paper using Cya-nex 272, Ionquest 801, and their mixtures. It was found that they performed very similarly in zinc selectivity over cobalt. Cyanex 272 performed much better than Ionquest 801 in cobalt separation from nickel. Citation: Liu, W.; Zhang, J.; Xu, Z.; However, very good separation of them was also obtained with Ionquest 801 at its low concentration Liang, J.; Zhu, Z.