OCT in Papilledema: What Am I Missing? 1. the Attendee Will Be Able to Describe the Problems with Subjective Grading of Papilled
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Post-Cataract Cystoid Macular Oedema Prevention – Update 2019
Review Cystoid Macular Oedema Post-cataract Cystoid Macular Oedema Prevention – Update 2019 Andrzej Grzybowski,1,2 Reda Zemaitiene,3 Lina Mikalauskiene3 1. Department of Ophthalmology, University of Warmia and Mazury, Olsztyn, Poland; 2. Institute for Research in Ophthalmology, Foundation for Ophthalmology Development, Poznan, Poland; 3. Department of Ophthalmology, Medical Academy, Lithuanian University of Health Sciences, Kaunas, Lithuania DOI: https://doi.org/10.17925/EOR.2019.13.1.37 seudophakic cystoid macular oedema (PCMO) is a common complication following both uncomplicated and complicated cataract surgery, becoming apparent about 6 weeks following surgery. PCMO may be asymptomatic in some cases, but in others is associated Pwith a reduction in visual acuity. The pathogenesis of PCMO is linked to postoperative inflammation and the release of inflammatory mediators. The use of topical steroids and/or nonsteroidal anti-inflammatory drugs can reduce the adverse effects of inflammation and have been used for the prevention and treatment of PCMO. However, the therapeutic effectiveness of these drugs is currently not well understood, partially because PCMO can spontaneously resolve as well as the multiple treatment protocols and the paucity of robust data exist. In this review we compare the various prophylactic options for PCMO and provide commentary on their efficacy. Keywords Various options for the prevention of pseudophakic cystoid macular oedema (PCMO) have been Pseudophakic cystoid macular oedema, offered. Nonsteroidal anti-inflammatory drugs (NSAIDs) seem to be beneficial in preventing post-operative inflammation, cataract surgery, postoperative inflammation; however, there is lack of evidence for long-term benefit after cataract nonsteroidal anti-inflammatory drugs surgery. What is more, topical NSAID preparations are difficult to compare, as studies differ in Disclosure: Andrzej Grzybowski, Reda inclusion criteria, patient characteristics, prescription and duration of treatment. -

The Usefulness of Systemic Inflammatory Markers As Diagnostic
A RQUIVOS B RASILEIROS DE ORIGINAL ARTICLE The usefulness of systemic inflammatory markers as diagnostic indicators of the pathogenesis of diabetic macular edema A utilidade de marcadores inflamatórios sistêmicos como indicadores diagnósticos da patogênese do edema macular diabético Cagri Ilhan1 , Mehmet Citirik2, Mehmet Murat Uzel3, Hasan Kiziltoprak4, Kemal Tekin5 1. Department of Ophthalmology, Hatay State Hospital, Hatay, Turkey. 2. Department of Ophthalmology, University of Health Sciences, Ankara Ulucanlar Eye Education and Research Hospital, Ankara, Turkey. 3. Department of Ophthalmology, Balikesir University, Balikesir, Turkey. 4. Department of Ophthalmology, Bingol Maternity and Child Hospital, Bingol, Turkey. 5. Department of Ophthalmology, Ercis State Hospital, Van, Turkey. ABSTRACT | Purpose: To investigate the usefulness of sys- groups were similar (diabetic macular edema vs. non-diabetic temic inflammatory markers [i.e., white blood cell and platelet macular edema, p=0.08; diabetic macular edema vs. control, counts, mean platelet volume, and their ratios] as diagnostic p=0.02; and non- diabetic macular edema vs. control, p=0.78). markers of the pathogenesis of diabetic macular edema. Me- All other parameters were similar between groups (all p>0.05). thods: The study cohort included 80 diabetic macular edema Conclusion: The neutrophil/lymphocyte ratio and mean platelet patients (40 with diabetic retinopathy and 40 without) and 40 volume of the diabetic macular edema group were higher than healthy age- and sex-matched controls. Neutrophil, lymphocyte, those of the non-diabetic macular edema and control groups. monocyte, and platelet counts, and the mean platelet volume A neutrophil/lymphocyte ratio cutoff value of ≥2.26 was identified were determined from peripheral blood samples, and the as an indicator of the pathogenesis of diabetic macular edema monocyte/lymphocyte, platelet/lymphocyte, and mean platelet with high sensitivity and specificity. -

Meeting Materials
BUSINESS, CONSUMER SERVICES, AND HOUSING AGENCY EDMUND G. BROWN JR., GOVERNOR STATE BOARD OF OPTOMETRY 2450 DEL PASO ROAD, SUITE 105, SACRAMENTO, CA 95834 0 P (916) 575-7170 F (916) 575-7292 www.optometry .ca.gov OPToMi fikY Continuing Education Course Approval Checklist Title: Provider Name: ☐Completed Application Open to all Optometrists? ☐ Yes ☐No Maintain Record Agreement? ☐ Yes ☐No ☐Correct Application Fee ☐Detailed Course Summary ☐Detailed Course Outline ☐PowerPoint and/or other Presentation Materials ☐Advertising (optional) ☐CV for EACH Course Instructor ☐License Verification for Each Course Instructor Disciplinary History? ☐Yes ☐No BUSINESS, CONSUMER SERVICES, AND HOUSING AGENCY GOVERNOR EDMUND G. BROWN JR. ~~ TATE BOARD OF OPTOMETRY }I /~E{:fLi\1 ~1' DELWSO ROAD, SUITE 105, SACRAMENTO, CA 95834 op'i,otii~l~ 1~0-A~ifiF' t\,rffi-7170 F (916) 575-7292 www.optometry.ca.gov EDUCATION COU Rw1t;--ftf""~'-!-J/-i~--,--__:___::...:..::....::~-~ $50 Mandatory Fee APPLICATION ,-~Jg l Pursuant to California Code of Regulations (CCR) § 1536, the Board will app~romv~e~c~ott=nfli1~nuFTT1t;tngn'zeWl:uc~rRif'tf-:~MT~~=ilt,;;,,_J receiving the applicable fee, the requested information below and it has been determined that the course meets criteria specified in CCR § 1536(g). In addition to the information requested below, please attach a copy of the course schedule, a detailed course outline and presentation materials (e.g., PowerPoint presentation). Applications must be submitted 45 days prior to the course presentation date. Please type or print -

Optical Coherence Tomography Demonstrates Subretinal Macular Edema from Papilledema
CLINICAL SCIENCES Optical Coherence Tomography Demonstrates Subretinal Macular Edema From Papilledema Vincent J. Hoye III, MD; Audina M. Berrocal, MD; Thomas R. Hedges III, MD; Maria Luz Amaro-Quireza, OD Objective: To evaluate macular changes in eyes with duction in visual acuity. The subretinal fluid appeared papilledema from increased intracranial pressure using to arise from the peripapillary region, and all showed optical coherence tomography (OCT). some improvement in central vision as the fluid re- solved. Methods: Fifty-five patients with papilledema seen dur- ing 1998 and 1999 were studied with OCT of the optic Conclusions: Subretinal fluid accumulations can cause nerve and retinal nerve fiber layer. Nineteen of these also decreased visual acuity in patients with papilledema. Op- had OCT of the macula during periods of acute, sub- tical coherence tomography can demonstrate subretinal acute, or recurrent papilledema and were evaluated in fluid and can be used to follow the course of this impor- detail for this report. tant visual complication of papilledema. Results: Seven patients had OCT evidence of sub- retinal fluid involving the macula. All had some re- Arch Ophthalmol. 2001;119:1287-1290 OSS OF visual acuity from pap- mal findings on neuroimaging scans and illedema or optic nerve head elevated opening pressures on lumbar swelling due to increased in- puncture. Two patients had brain metas- tracranial pressure is uncom- tases, one from breast cancer and the other mon. The visual conse- from testicular cancer. Six patients were Lquences of papilledema usually are limited treated medically with acetazolamide to visual field defects and enlargement of and/or furosemide. -

National Eye Institute Visual Function Questionnaire (NEI- VFQ25) in Subjects with IIH and Normal Controls
Title: Correlation of Visual Function and National Eye Institute Visual Function Questionnaire (NEI- VFQ25) in subjects with IIH and normal controls. Melanie Truong, DO OD Introduction and Purpose Aims of the study: 1. To assess contrast sensitivity acuity and rapid eye movements (saccades) as a measure of visual function in Idiopathic Intracranial Hypertension (IIH) patients compared to normal patients using the King-Devick Variable Contrast Acuity Chart and the K-D rapid eye movement. 2. To assess subjects quality of visual function using the National Eye Institute Visual Function Questionnaire (NEI-VFQ25) questionnaire and Supplement as a comparison between IIH subjects and normal controls. 3. To study the correlation between visual function and quality of visual function questionnaire in subjects with IIH compared to normal controls. Contrast sensitivity is a measure of afferent visual system. Contrast sensitivity deficit with preservation of normal Snellen acuity has also been reported in glaucoma, compressive disorders of the anterior visual pathways, retinal diseases, and with cerebral lesions.1 This test is significantly more sensitive than Snellen acuity .1 It is also superior for serial testing in patients as there was significant improvement in contrast scores and papilledema grade but no significant change in Snellen acuity.1 Visual manifestation of IIH can also include parafoveal deficits. This can lead to deficits in spatial frequency contrast sensitivity.1 Contrast sensitivity is abnormal initially and improved with regression of papilledema.2 Since decisions on therapy in IIH are based on the presence and change in visual function, assessing visual acuities is not the most accurate measure of visual status in IIH. -

Central Serous Papillopathy by Optic Nerve Head Drusen
Clinical Ophthalmology Dovepress open access to scientific and medical research Open Access Full Text Article CASE REPORT Central serous papillopathy by optic nerve head drusen Ana Marina Suelves1 Abstract: We report a 38-year-old man with a complaint of blurred vision in his right eye for the Ester Francés-Muñoz1 previous 5 days. He had bilateral optic disc drusen. Fluorescein angiography revealed multiple Roberto Gallego-Pinazo1 hyperfluorescent foci within temporal optic discs and temporal inferior arcade in late phase. Diamar Pardo-Lopez1 Optical coherence tomography showed bilateral peripapillary serous detachment as well as right Jose Luis Mullor2 macular detachment. This is the first reported case of a concurrent peripapillary and macular Jose Fernando Arevalo3 detachment in a patient with central serous papillopathy by optic disc drusen. Central serous papillopathy is an atypical form of central serous chorioretinopathy that should be considered Manuel Díaz-Llopis1,4,5 as a potential cause of acute loss of vision in patients with optic nerve head drusen. 1 Department of Ophthalmology, La Fe Keywords: central serous papillopathy, peripapillary central serous chorioretinopathy, optic University Hospital, Valencia, Spain; For personal use only. 2Instituto de Investigación Sanitaria, nerve head drusen, peripapillary subretinal fluid Fundación para la investigación, La Fe Hospital, Valencia, Spain; 3Retina and vitreous service, Clínica Introduction Oftalmológica Centro Caracas, Optic nerve head drusen (ONHD) are hyaline material calcificated -
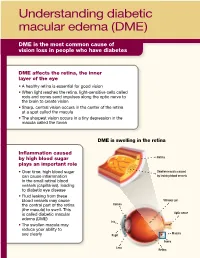
Understanding Diabetic Macular Edema (DME)
Understanding diabetic macular edema (DME) DME is the most common cause of vision loss in people who have diabetes DME affects the retina, the inner layer of the eye • A healthy retina is essential for good vision • When light reaches the retina, light-sensitive cells called rods and cones send impulses along the optic nerve to the brain to create vision • Sharp, central vision occurs in the center of the retina at a spot called the macula • The sharpest vision occurs in a tiny depression in the macula called the fovea DME is swelling in the retina Inflammation caused by high blood sugar Retina plays an important role • Over time, high blood sugar Swollen macula caused can cause inflammation by leaking blood vessels in the small retinal blood vessels (capillaries), leading to diabetic eye disease • Fluid leaking from these blood vessels may cause Vitreous gel Cornea the central part of the retina (the macula) to swell. This Optic nerve is called diabetic macular edema (DME) Iris • The swollen macula may reduce your ability to Macula see clearly Pupil Fovea Lens Retina Understanding DME (continued) Symptoms of DME • Early DME may not have any symptoms • As DME worsens, it can cause blurry central vision ranging from mild to severe • DME can cause significant vision loss over time How DME can affect vision Mild blurriness Moderate blurriness Severe blurriness Untreated DME can lead to vision loss • Talk to your doctor about treatment options • If you develop cataracts (hazy spots that can affect vision) in the lenses of your eyes, the natural lenses may be surgically removed and replaced with artificial ones • Having cataracts or artificial lenses can affect your treatment options ©2014 Allergan, Inc., Irvine, CA 92612 APC67SH14 141256. -

Idiopathic Intracranial Hypertension
IDIOPATHIC INTRACRANIAL HYPERTENSION William L Hills, MD Neuro-ophthalmology Oregon Neurology Associates Affiliated Assistant Professor Ophthalmology and Neurology Casey Eye Institute, OHSU No disclosures CASE - 19 YO WOMAN WITH HEADACHES X 3 MONTHS Headaches frontal PMHx: obesity Worse lying down Meds: takes ibuprofen for headaches Wake from sleep Pulsatile tinnitus x 1 month. Vision blacks out transiently when she bends over or sits down EXAMINATION Vision: 20/20 R eye, 20/25 L eye. Neuro: PERRL, no APD, EOMI, VF full to confrontation. Dilated fundoscopic exam: 360 degree blurring of disc margins in both eyes, absent SVP. Formal visual field testing: Enlargement of the blind spot, generalized constriction both eyes. MRI brain: Lumbar puncture: Posterior flattening of Opening pressure 39 the globes cm H20 Empty sella Normal CSF studies otherwise normal Headache improved after LP IDIOPATHIC INTRACRANIAL HYPERTENSION SYNDROME: Increased intracranial pressure without ventriculomegaly or mass lesion Normal CSF composition NOMENCLATURE Idiopathic intracranial hypertension (IIH) Benign intracranial hypertension Pseudotumor cerebri Intracranial hypertension secondary to… DIAGNOSTIC CRITERIA Original criteria have been updated to reflect new imaging modalities: 1492 Friedman and Jacobsen. Neurology 2002; 59: Symptoms and signs reflect only those of - increased ICP or papilledema 1495 Documented increased ICP during LP in lateral decubitus position Normal CSF composition No evidence of mass, hydrocephalus, structural -

Amaurosis Fugax (Transient Monocular Or Binocular Vision Loss)
Amaurosis fugax (transient monocular or binocular vision loss) Syndee Givre, MD, PhD Gregory P Van Stavern, MD The next version of UpToDate (15.3) will be released in October 2007. INTRODUCTION AND DEFINITIONS — Amaurosis fugax (from the Greek "amaurosis," meaning dark, and the Latin "fugax," meaning fleeting) refers to a transient loss of vision in one or both eyes. Varied use of common terminology may cause some confusion when reading the literature. Some suggest that "amaurosis fugax" implies a vascular cause for the visual loss, but the term continues to be used when describing visual loss from any origin and involving one or both eyes. The term "transient monocular blindness" is also often used but is not ideal, since most patients do not experience complete loss of vision with the episode. "Transient monocular visual loss" (TMVL) and "transient binocular visual loss" (TBVL) are preferred to describe abrupt and temporary loss of vision in one or both eyes, since they carry no connotation regarding etiology. Transient visual loss, either monocular or binocular, reflects a heterogeneous group of disorders, some relatively benign and others with grave neurologic or ophthalmologic implications. The task of the clinician is to use the history and examination to localize the problem to a region in the visual pathways, identify potential etiologies, and, when indicated, perform a focused battery of laboratory tests to confirm or exclude certain causes. Therapeutic interventions and prognostic implications are specific to the underlying cause. This topic discusses transient visual loss. Other ocular and cerebral ischemic syndromes are discussed separately. APPROACH TO TRANSIENT VISUAL LOSS — By definition, patients with transient visual loss almost always present after the episode has resolved; hence, the neurologic and ophthalmologic examination is usually normal. -

Polycythemia Vera Presenting with Bilateral Papilledema Retinitis
July - August 2009 Lett ers to the Editor 325 Amjad Salman, Pragya Parmar, vary signiÞ cantly. With the increasing use of MRI in all cases Vanila G Coimbatore, Rajmohan Meenakshisunderam, suspected to be a brain syndrome, CVT has been increasing Nelson Jesudasan A Christdas diagnosed. MRI is now the gold standard in the diagnosis of CVT as rightly done in this case. Institute of Ophthalmology, Joseph Eye Hospital, Tiruchirapalli - 620 001, India Visual loss in CVT maybe due to thrombotic ischemia of any structure of the visual pathway or due to pressure on the optic Correspondence to Dr. Salman Amjad, Institute of Ophthalmology, Joseph Eye Hospital, Tiruchirapalli - 620 001, India. nerve due to the transmitt ed raised intracranial pressure (ICP). E-mail: [email protected] All cases of CVT with visual loss require visual Þ eld analysis and measurement of optic nerve sheath diameter using B-scan References ultrasonography (USG). Visual loss in patients with CVT due to transmitt ed raised ICP (indicated by increased optic nerve sheath 1. Gillies MC, Simpson JM, Billson FA, Luo W, Penfold P, Chua W, et al. diameter on USG) not amenable to medical management is an Safety of an intravitreal injection of triamcinolone: Results from a indication for optic nerve sheath decompression (ONSD). ONSD randomized clinical trial. Arch Ophthalmol 2004;122:336-40. as a treatment option for the visual loss in the left eye should have 2. Thompson JT. Cataract formation and other complications of intravitreal triamcinolone acetonide for macular edema. Am J been off ered to the patient in this case, as it has been shown to [4,5] Ophthalmol 2006;141:629-37. -

Introduction to Micropulse for Central Serous Chorioretinopathy
Advertorial MicroPulse for Central Serous Chorioretinopathy Sponsored by Quantel Medical Introduction to MicroPulse for Central Serous Chorioretinopathy MicroPulse laser has become one of our hospital’s standard treatments for CSCR. BY PROF. DR. SASCHA FAUSER, MD I would like to introduce you to the principle of One of the challenges with MicroPulse laser treatment is MicroPulse laser therapy. that you cannot visualize any laser effect during the treatment, To understand MicroPulse laser technology, we must which could give you the feeling that the treatment is not effec- first understand conventional photocoagulation. tive. Overall, the problem is overtreating, but undertreatment With conventional photocoagulation, the laser trans- can also be an issue. To avoid undertreating, surgeons should fers energy to the retina. The longer the laser beam is on, the more apply dense treatment and focus the laser carefully. The yel- heat is generated. This heat may result in tissue damage by coagu- low 577 nm wavelength has an advantage in these situations lating the proteins. It is similar to how an egg loses its transparency because, due to its absorption characteristics, it can be titrated. when heat is applied to it. Basically, you apply the laser to the periphery of the edematous We do not yet know the exact mechanism of action of laser, but area (in a nonedematous area) on low power that is increased one hypothesis is that the damaged photoreceptors are replaced by gradually until a just-visible effect appears. Once the thermal glial cells, which have less oxygen consumption, less vascular endo- threshold is determined for a patient, you reduce the power to thelial growth factor, less edema, and less neovascularization. -

Papilledema": Neuroradiologic Evaluation of Optic Disk Protrusion with Dynamic Orbital CT
681 "Papilledema": Neuroradiologic Evaluation of Optic Disk Protrusion with Dynamic Orbital CT John R. Jinkins 1 Current-generation CT scc·lners enable the visualization in vivo of structures and substructures that were previously unobservable. Certainly the orbit and optic nerve/ sheath complex have demonstrated a great number of pathologic and normal anatomic variations. It has been found in patients with elevated intracranial pressure that what was previously thought to be simple papilledema in fact masks a surprisingly large component of optic papilla protru<;ion, There may be a variable amount of increased intercellular/axonal fluid within tt.. - optic disk in patients with increased intracranial pressure; however, a significant factor in the " swollen disk" is the simple transmission of pressure along the optic nerve sheath to the papilla, causing it to bulge. Further investigations with dynamic CT reveal that there is decreased perfusion of the optic disk in the active phase of severe increased intracranial pressure in patients with papilledema and/or protrusion as compared with normal control subjects. This de pressed flow pattern seems to originate subacutely and appears to resolve in certain patients after normalization of the elevated pressure. These findings apparently indicate that clinical intervention in cases of intracranial hypertension to restore the hemodynamic status of the optic disk would be timely, and thereby avert irreversible damage. This suggests and supports the theory that increased intracranial pressure may lead to rapid vision loss by the mechanical mechanism of pressure projected directly to the junction of the optic nerve and optic nerve head, leading to decreased perfusion, ischemia, axonal flow stasis, and resultant optic nerve atrophy.