The Effects of Habitat Parameters on the Behavior, Ecology, and Conservation of the Udzungwa Red Colobus Monkey
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Saving Habitats Saving Species Since 1989 Inside This
WLTnews ISSUE No. 61 SPRING 2019 Inside this issue: • £100 an acre: new opportunity in Jungle for Jaguars corridor • News in pictures: conservation stories from around the world • Field reports from Paraguay and Vietnam Saving habitats Saving species since 1989 worldlandtrust.org 1 New opportunity in the Jungle for Jaguars corridor Buy an acre of tropical forest for £100 COUNTRY: BELIZE LAND: 1,818 ACRES TARGET: £181,800 The success of our Jungle for Jaguars appeal has unlocked an opportunity to buy an additional piece of forest to add to the corridor. And the good news is that it is available for purchase at our Buy an Acre price of £100 per acre. The Jungle for Jaguars appeal in late 2018 was an incredible success. World Land Trust (WLT) supporters raised £600,000 for the purchase and protection of 8,154 acres in a vital wildlife corridor. This land has now been purchased by our partner the Corozal Sustainable Future Initiative (CSFI), who manage several of the protected areas in this corridor. The additional 1,818 acres would ensure safe passage for Jaguars on the eastern side of Shipstern Lagoon. Within this parcel of land live the Fireburn Community who have 187 acres of community lands. CSFI will be building trust and 1,818 acres for purchase engagement with them as well as helping Shipstern Lagoon to develop sustainable livelihoods. Protected areas Buy an Acre Donate online (worldlandtrust.org), Community land by phone (01986 874422) or by post (using the enclosed donation form). Roseate Spoonbill Shipstern Lagoon, with its mangrove islands and water bird colonies, is the largest inland lagoon in Belize. -

Activity and Ranging Patterns of Guerezas in the Kakamega Forest: Intergroup Variation and Implications for Intragroup Feeding Competition
P1: Vendor International Journal of Primatology [ijop] PP095-296772 June 6, 2001 11:50 Style file version Nov. 19th, 1999 International Journal of Primatology, Vol. 22, No. 4, 2001 Activity and Ranging Patterns of Guerezas in the Kakamega Forest: Intergroup Variation and Implications for Intragroup Feeding Competition Peter J. Fashing1,2 Received November 29, 1999; revision July 13, 2000; accepted August 10, 2000 From March 1997 to February 1998, I investigated the activity patterns of 2 groups and the ranging patterns of 5 groups of eastern black-and-white colobus (Colobus guereza), aka guerezas, in the Kakamega Forest, Kenya. Guerezas at Kakamega spent more of their time resting than any other pop- ulation of colobine monkeys studied to date. In addition, I recorded not one instance of intragroup aggression in 16,710 activity scan samples, providing preliminary evidence that intragroup contest competition may be rare or ab- sent among guerezas at Kakamega. Mean daily path lengths ranged from 450 to 734 m, and home range area ranged from 12 to 20 ha, though home range area may have been underestimated for several of the study groups. Home range overlap was extensive with 49–83% of each group’s range overlapped by the ranges of other groups. Despite the high level of home range overlap, the frequently entered areas (quadrats entered on 30% of a group’s total study days) of any one group were not frequently entered by any other study group. Mean daily path length is not significantly correlated with levels of availability or consumption of any plant part item. -

Habitat Utilization and Feeding Biology of Himalayan Grey Langur
动 物 学 研 究 2010,Apr. 31(2):177−188 CN 53-1040/Q ISSN 0254-5853 Zoological Research DOI:10.3724/SP.J.1141.2010.02177 Habitat Utilization and Feeding Biology of Himalayan Grey Langur (Semnopithecus entellus ajex) in Machiara National Park, Azad Jammu and Kashmir, Pakistan Riaz Aziz Minhas1,*, Khawaja Basharat Ahmed2, Muhammad Siddique Awan2, Naeem Iftikhar Dar3 (1. World Wide Fund for Nature-Pakistan (WWF-Pakistan) AJK Office Muzaffarabad, Azad Jammu and Kashmir 13100, Pakistan; 2. Department ofZoology, University of Azad Jammu and Kashmir Muzaffarabad, Azad Jammu and Kashmir 13100, Pakistan; 3. Department of Wildlife and Fisheries, Government of Azad Jammu and Kashmir, Muzaffarabad, Azad Jammu and Kashmir 13100, Pakistan) Abstract: Habitat utilization and feeding biology of Himalayan Grey Langur (Semnopithecus entellus ajex) were studied from April, 2006 to April, 2007 in Machiara National Park, Azad Jammu and Kashmir, Pakistan. The results showed that in the winter season the most preferred habitat of the langurs was the moist temperate coniferous forests interspersed with deciduous trees, while in the summer season they preferred to migrate into the subalpine scrub forests at higher altitudes. Langurs were folivorous in feeding habit, recorded as consuming more than 49 plant species (27 in summer and 22 in winter) in the study area. The mature leaves (36.12%) were preferred over the young leaves (27.27%) while other food components comprised of fruits (17.00%), roots (9.45%), barks (6.69%), flowers (2.19%) and stems (1.28%) of various plant species. Key words: Himalayan Grey Langur; Habitat; Food biology; Machiara National Park 喜马拉雅灰叶猴栖息地利用和食性生物学 Riaz Aziz Minhas1,*, Khawaja Basharat Ahmed2, Muhammad Siddique Awan2, Naeem Iftikhar Dar3 (1. -

Annual Review 2019 Registered Charity 1001291
ANNUAL REVIEW 2019 REGISTERED CHARITY 1001291 worldlandtrust.org A YEAR TO REMEMBER A FEW WORDS FROM Dr Gerard Bertrand Steve Backshall, MBE Hon President, WLT Patron For WLT so many years of the last three decades have been marked by One Wild Night 2019 outstanding progress in conservation. In December, Steve Backshall hosted This past year, however, was a magnificent evening at the Royal remarkable because the Trust recorded Geographical Society. its largest number and diversity of Playing to a packed house, Steve conservation projects throughout the welcomed on stage some of the UK’s world. The Trust truly is making a leading adventurers, explorers and difference for wildlife from Southeast sports personalities including his wife, Asia to Africa to the Americas. Helen Glover, Baroness Tanni Grey- the Río Zuñac appeal we launched on the Thompson, Dan Snow, Professor Ben evening to purchase cloud forest in It was also the year of successful Garrod, Phoebe Smith and Dwayne Ecuador has reached its fundraising transition from our incomparable Fields. Monty Don compered the target. Because of your support, WLT and founders John and Viv Burton to new evening and young environmental Fundación EcoMinga can expand the Trust leadership. The Trust is fortunate activist, Bella Lack spoke passionately reserve, securing it for the species that to have attracted an outstanding about the threats facing the planet and live here. conservationist, Dr. Jonathan Barnard as solutions within our grasp. We all know that we need to do the new Chief Executive to lead WLT. something to protect our planet. I believe The past three decades seem to have Steve says: the most practical step we can take is to flown by since John Burton and I “One Wild Night was filled with inspiring continue purchasing these remote hatched the plan to build a new stories of conservation and adventure in wildernesses, placing them in the hands organisation in the UK to support the wild world. -

The Role of Exposure in Conservation
Behavioral Application in Wildlife Photography: Developing a Foundation in Ecological and Behavioral Characteristics of the Zanzibar Red Colobus Monkey (Procolobus kirkii) as it Applies to the Development Exhibition Photography Matthew Jorgensen April 29, 2009 SIT: Zanzibar – Coastal Ecology and Natural Resource Management Spring 2009 Advisor: Kim Howell – UDSM Academic Director: Helen Peeks Table of Contents Acknowledgements – 3 Abstract – 4 Introduction – 4-15 • 4 - The Role of Exposure in Conservation • 5 - The Zanzibar Red Colobus (Piliocolobus kirkii) as a Conservation Symbol • 6 - Colobine Physiology and Natural History • 8 - Colobine Behavior • 8 - Physical Display (Visual Communication) • 11 - Vocal Communication • 13 - Olfactory and Tactile Communication • 14 - The Importance of Behavioral Knowledge Study Area - 15 Methodology - 15 Results - 16 Discussion – 17-30 • 17 - Success of the Exhibition • 18 - Individual Image Assessment • 28 - Final Exhibition Assessment • 29 - Behavioral Foundation and Photography Conclusion - 30 Evaluation - 31 Bibliography - 32 Appendices - 33 2 To all those who helped me along the way, I am forever in your debt. To Helen Peeks and Said Hamad Omar for a semester of advice, and for trying to make my dreams possible (despite the insurmountable odds). Ali Ali Mwinyi, for making my planning at Jozani as simple as possible, I thank you. I would like to thank Bi Ashura, for getting me settled at Jozani and ensuring my comfort during studies. Finally, I am thankful to the rangers and staff of Jozani for welcoming me into the park, for their encouragement and support of my project. To Kim Howell, for agreeing to support a project outside his area of expertise, I am eternally grateful. -
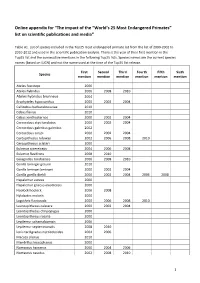
Online Appendix for “The Impact of the “World's 25 Most Endangered
Online appendix for “The impact of the “World’s 25 Most Endangered Primates” list on scientific publications and media” Table A1. List of species included in the Top25 most endangered primate list from the list of 2000-2002 to 2010-2012 and used in the scientific publication analysis. There is the year of their first mention in the Top25 list and the consecutive mentions in the following Top25 lists. Species names are the current species names (based on IUCN) and not the name used at the time of the Top25 list release. First Second Third Fourth Fifth Sixth Species mention mention mention mention mention mention Ateles fusciceps 2006 Ateles hybridus 2006 2008 2010 Ateles hybridus brunneus 2004 Brachyteles hypoxanthus 2000 2002 2004 Callicebus barbarabrownae 2010 Cebus flavius 2010 Cebus xanthosternos 2000 2002 2004 Cercocebus atys lunulatus 2000 2002 2004 Cercocebus galeritus galeritus 2002 Cercocebus sanjei 2000 2002 2004 Cercopithecus roloway 2002 2006 2008 2010 Cercopithecus sclateri 2000 Eulemur cinereiceps 2004 2006 2008 Eulemur flavifrons 2008 2010 Galagoides rondoensis 2006 2008 2010 Gorilla beringei graueri 2010 Gorilla beringei beringei 2000 2002 2004 Gorilla gorilla diehli 2000 2002 2004 2006 2008 Hapalemur aureus 2000 Hapalemur griseus alaotrensis 2000 Hoolock hoolock 2006 2008 Hylobates moloch 2000 Lagothrix flavicauda 2000 2006 2008 2010 Leontopithecus caissara 2000 2002 2004 Leontopithecus chrysopygus 2000 Leontopithecus rosalia 2000 Lepilemur sahamalazensis 2006 Lepilemur septentrionalis 2008 2010 Loris tardigradus nycticeboides -

REVIEW ARTICLE Agroecosystems and Primate Conservation in the Tropics: a Review
American Journal of Primatology 74:696–711 (2012) REVIEW ARTICLE Agroecosystems and Primate Conservation in The Tropics: A Review ∗ ALEJANDRO ESTRADA1 , BECKY E. RABOY2,3, AND LEONARDO C. OLIVEIRA3-6 1Estaci´on de Biolog´ıa Tropical Los Tuxtlas Instituto de Biolog´ıa, Universidad Nacional Aut´onoma de M´exico, Mexico City, Mexico 2Conservation Ecology Center, Smithsonian Conservation Biology Institute, National Zoological Park, Washington, DC 3Instituto de Estudos S´ocioambientais do Sul da Bahia (IESB), Ilh´eus-BA, Brazil 4Programa de P´os-Gradua¸c˜ao em Ecologia, Universidade Federal do Rio de Janeiro, Rio de Janeiro, Brazil 5Programa de P´os-Gradua¸c˜ao em Ecologia e Conserva¸c˜ao da Biodiversidade, Universidade Estadual de Santa Cruz, Ilh´eus-BA, Brazil 6Bicho do Mato Instituto de Pesquisa, Belo Horizonte-MG, Brazil Agroecosystems cover more than one quarter of the global land area (ca. 50 million km2) as highly simplified (e.g. pasturelands) or more complex systems (e.g. polycultures and agroforestry systems) with the capacity to support higher biodiversity. Increasingly more information has been published about primates in agroecosystems but a general synthesis of the diversity of agroecosystems that primates use or which primate taxa are able to persist in these anthropogenic components of the landscapes is still lacking. Because of the continued extensive transformation of primate habitat into human-modified landscapes, it is important to explore the extent to which agroecosystems are used by primates. In this article, we reviewed published information on the use of agroecosystems by primates in habitat countries and also discuss the potential costs and benefits to human and nonhuman primates of primate use of agroecosystems. -

Science Journals
SCIENCE ADVANCES | REVIEW PRIMATOLOGY 2017 © The Authors, some rights reserved; Impending extinction crisis of the world’s primates: exclusive licensee American Association Why primates matter for the Advancement of Science. Distributed 1 2 3 4 under a Creative Alejandro Estrada, * Paul A. Garber, * Anthony B. Rylands, Christian Roos, Commons Attribution 5 6 7 7 Eduardo Fernandez-Duque, Anthony Di Fiore, K. Anne-Isola Nekaris, Vincent Nijman, NonCommercial 8 9 10 10 Eckhard W. Heymann, Joanna E. Lambert, Francesco Rovero, Claudia Barelli, License 4.0 (CC BY-NC). Joanna M. Setchell,11 Thomas R. Gillespie,12 Russell A. Mittermeier,3 Luis Verde Arregoitia,13 Miguel de Guinea,7 Sidney Gouveia,14 Ricardo Dobrovolski,15 Sam Shanee,16,17 Noga Shanee,16,17 Sarah A. Boyle,18 Agustin Fuentes,19 Katherine C. MacKinnon,20 Katherine R. Amato,21 Andreas L. S. Meyer,22 Serge Wich,23,24 Robert W. Sussman,25 Ruliang Pan,26 27 28 Inza Kone, Baoguo Li Downloaded from Nonhuman primates, our closest biological relatives, play important roles in the livelihoods, cultures, and religions of many societies and offer unique insights into human evolution, biology, behavior, and the threat of emerging diseases. They are an essential component of tropical biodiversity, contributing to forest regeneration and ecosystem health. Current information shows the existence of 504 species in 79 genera distributed in the Neotropics, mainland Africa, Madagascar, and Asia. Alarmingly, ~60% of primate species are now threatened with extinction and ~75% have de- clining populations. This situation is the result of escalating anthropogenic pressures on primates and their habitats— mainly global and local market demands, leading to extensive habitat loss through the expansion of industrial agri- http://advances.sciencemag.org/ culture, large-scale cattle ranching, logging, oil and gas drilling, mining, dam building, and the construction of new road networks in primate range regions. -

World's Most Endangered Primates
Primates in Peril The World’s 25 Most Endangered Primates 2016–2018 Edited by Christoph Schwitzer, Russell A. Mittermeier, Anthony B. Rylands, Federica Chiozza, Elizabeth A. Williamson, Elizabeth J. Macfie, Janette Wallis and Alison Cotton Illustrations by Stephen D. Nash IUCN SSC Primate Specialist Group (PSG) International Primatological Society (IPS) Conservation International (CI) Bristol Zoological Society (BZS) Published by: IUCN SSC Primate Specialist Group (PSG), International Primatological Society (IPS), Conservation International (CI), Bristol Zoological Society (BZS) Copyright: ©2017 Conservation International All rights reserved. No part of this report may be reproduced in any form or by any means without permission in writing from the publisher. Inquiries to the publisher should be directed to the following address: Russell A. Mittermeier, Chair, IUCN SSC Primate Specialist Group, Conservation International, 2011 Crystal Drive, Suite 500, Arlington, VA 22202, USA. Citation (report): Schwitzer, C., Mittermeier, R.A., Rylands, A.B., Chiozza, F., Williamson, E.A., Macfie, E.J., Wallis, J. and Cotton, A. (eds.). 2017. Primates in Peril: The World’s 25 Most Endangered Primates 2016–2018. IUCN SSC Primate Specialist Group (PSG), International Primatological Society (IPS), Conservation International (CI), and Bristol Zoological Society, Arlington, VA. 99 pp. Citation (species): Salmona, J., Patel, E.R., Chikhi, L. and Banks, M.A. 2017. Propithecus perrieri (Lavauden, 1931). In: C. Schwitzer, R.A. Mittermeier, A.B. Rylands, F. Chiozza, E.A. Williamson, E.J. Macfie, J. Wallis and A. Cotton (eds.), Primates in Peril: The World’s 25 Most Endangered Primates 2016–2018, pp. 40-43. IUCN SSC Primate Specialist Group (PSG), International Primatological Society (IPS), Conservation International (CI), and Bristol Zoological Society, Arlington, VA. -

Project Update: August 2002 Habitat Quality and Fragmentation and The
Project Update: August 2002 Habitat Quality and Fragmentation and the Distribution and Abundance of the Tana River Red Colobus Monkey, Procolobus Rufomitratus, in Eastern Kenya Habitat fragmentation constitutes the most significant threat to survival of primates in the world and therefore there is an urgent need for effective conservation measures that protect primates living in fragmented habitats. Few studies have investigated what habitat factors determine the probability that primates will occur in habitat fragments or effectiveness of specific conservation measures established to protect them. I analysed forest habitat and colobus population attributes to determine factors that influenced occurrence and abundance of the critically endangered Tana River red colobus in forest fragments in Kenya. The probability of colobus inhabiting forests was positively related to amount of habitat edge and tree species composition. Most of the variance in the density of colobus monkeys was explained by: basal area of food trees, basal area per food tree, density of food trees, and basal area per tree for all trees. Therefore effective primate conservation in fragmented habitats may require strategies that consider different factors that influence both the occurrence of a species and its abundance. To determine whether the Tana River Primate National Reserve is effective in conserving the Tana River red colobus, and by extension other species, I determined whether colobus population attributes or forest habitat attributes differed between inside and outside the reserve. I found no difference in colobus density or mean group size between forests inside and outside the reserve. Forests outside the reserve had significantly higher total basal area of trees, mean basal area per tree, and total basal area of stumps left by trees cut for human use. -

Influence of External and Internal Environmental Factors on Intestinal Microbiota of Wild and Domestic Animals A
Influence of external and internal environmental factors on intestinal microbiota of wild and domestic of animals factors on intestinal microbiota Influence of external and internal environmental Influence of external and internal environmental factors on intestinal microbiota of wild and domestic animals A. Umanets Alexander Umanets Propositions 1. Intestinal microbiota and resistome composition of wild animals are mostly shaped by the animals’ diet and lifestyle. (this thesis) 2. When other environmental factors are controlled, genetics of the host lead to species- or breed specific microbiota patterns. (this thesis) 3. Identifying the response of microbial communities to factors that only have a minor contribution to overall microbiota variation faces the same problems as the discovery of exoplanets. 4. Observational studies in microbial ecology using cultivation- independent methods should be considered only as a guide for further investigations that employ controlled experimental conditions and mechanistic studies of cause-effect relationships. 5. Public fear of genetic engineering and artificial intelligence is not helped by insufficient public education and misleading images created through mass- and social media. 6. Principles of positive (Darwinian) and negative selection govern the repertoire of techniques used within martial arts. Propositions belonging to the thesis, entitled Influence of external and internal environmental factors on intestinal microbiota of wild and domestic animals Alexander Umanets Wageningen, 17 October -

Gastrointestinal Parasites of the Colobus Monkeys of Uganda
J. Parasitol., 91(3), 2005, pp. 569±573 q American Society of Parasitologists 2005 GASTROINTESTINAL PARASITES OF THE COLOBUS MONKEYS OF UGANDA Thomas R. Gillespie*², Ellis C. Greiner³, and Colin A. Chapman²§ Department of Zoology, University of Florida, Gainesville, Florida 32611. e-mail: [email protected] ABSTRACT: From August 1997 to July 2003, we collected 2,103 fecal samples from free-ranging individuals of the 3 colobus monkey species of UgandaÐthe endangered red colobus (Piliocolobus tephrosceles), the eastern black-and-white colobus (Co- lobus guereza), and the Angolan black-and-white colobus (C. angolensis)Ðto identify and determine the prevalence of gastro- intestinal parasites. Helminth eggs, larvae, and protozoan cysts were isolated by sodium nitrate ¯otation and fecal sedimentation. Coprocultures facilitated identi®cation of helminths. Seven nematodes (Strongyloides fulleborni, S. stercoralis, Oesophagostomum sp., an unidenti®ed strongyle, Trichuris sp., Ascaris sp., and Colobenterobius sp.), 1 cestode (Bertiella sp.), 1 trematode (Dicro- coeliidae), and 3 protozoans (Entamoeba coli, E. histolytica, and Giardia lamblia) were detected. Seasonal patterns of infection were not apparent for any parasite species infecting colobus monkeys. Prevalence of S. fulleborni was higher in adult male compared to adult female red colobus, but prevalence did not differ for any other shared parasite species between age and sex classes. Colobinae is a large subfamily of leaf-eating, Old World 19 from Angolan black-and-white colobus. Red colobus are sexually monkeys represented in Africa by species of 3 genera, Colobus, dimorphic, with males averaging 10.5 kg and females 7.0 kg (Oates et al., 1994); they display a multimale±multifemale social structure and Procolobus, and Piliocolobus (Grubb et al., 2002).