Quantities and Units 1
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Variables in Mathematics Education
Variables in Mathematics Education Susanna S. Epp DePaul University, Department of Mathematical Sciences, Chicago, IL 60614, USA http://www.springer.com/lncs Abstract. This paper suggests that consistently referring to variables as placeholders is an effective countermeasure for addressing a number of the difficulties students’ encounter in learning mathematics. The sug- gestion is supported by examples discussing ways in which variables are used to express unknown quantities, define functions and express other universal statements, and serve as generic elements in mathematical dis- course. In addition, making greater use of the term “dummy variable” and phrasing statements both with and without variables may help stu- dents avoid mistakes that result from misinterpreting the scope of a bound variable. Keywords: variable, bound variable, mathematics education, placeholder. 1 Introduction Variables are of critical importance in mathematics. For instance, Felix Klein wrote in 1908 that “one may well declare that real mathematics begins with operations with letters,”[3] and Alfred Tarski wrote in 1941 that “the invention of variables constitutes a turning point in the history of mathematics.”[5] In 1911, A. N. Whitehead expressly linked the concepts of variables and quantification to their expressions in informal English when he wrote: “The ideas of ‘any’ and ‘some’ are introduced to algebra by the use of letters. it was not till within the last few years that it has been realized how fundamental any and some are to the very nature of mathematics.”[6] There is a question, however, about how to describe the use of variables in mathematics instruction and even what word to use for them. -

An Atomic Physics Perspective on the New Kilogram Defined by Planck's Constant
An atomic physics perspective on the new kilogram defined by Planck’s constant (Wolfgang Ketterle and Alan O. Jamison, MIT) (Manuscript submitted to Physics Today) On May 20, the kilogram will no longer be defined by the artefact in Paris, but through the definition1 of Planck’s constant h=6.626 070 15*10-34 kg m2/s. This is the result of advances in metrology: The best two measurements of h, the Watt balance and the silicon spheres, have now reached an accuracy similar to the mass drift of the ur-kilogram in Paris over 130 years. At this point, the General Conference on Weights and Measures decided to use the precisely measured numerical value of h as the definition of h, which then defines the unit of the kilogram. But how can we now explain in simple terms what exactly one kilogram is? How do fixed numerical values of h, the speed of light c and the Cs hyperfine frequency νCs define the kilogram? In this article we give a simple conceptual picture of the new kilogram and relate it to the practical realizations of the kilogram. A similar change occurred in 1983 for the definition of the meter when the speed of light was defined to be 299 792 458 m/s. Since the second was the time required for 9 192 631 770 oscillations of hyperfine radiation from a cesium atom, defining the speed of light defined the meter as the distance travelled by light in 1/9192631770 of a second, or equivalently, as 9192631770/299792458 times the wavelength of the cesium hyperfine radiation. -

Kind of Quantity’
NIST Technical Note 2034 Defning ‘kind of quantity’ David Flater This publication is available free of charge from: https://doi.org/10.6028/NIST.TN.2034 NIST Technical Note 2034 Defning ‘kind of quantity’ David Flater Software and Systems Division Information Technology Laboratory This publication is available free of charge from: https://doi.org/10.6028/NIST.TN.2034 February 2019 U.S. Department of Commerce Wilbur L. Ross, Jr., Secretary National Institute of Standards and Technology Walter Copan, NIST Director and Undersecretary of Commerce for Standards and Technology Certain commercial entities, equipment, or materials may be identifed in this document in order to describe an experimental procedure or concept adequately. Such identifcation is not intended to imply recommendation or endorsement by the National Institute of Standards and Technology, nor is it intended to imply that the entities, materials, or equipment are necessarily the best available for the purpose. National Institute of Standards and Technology Technical Note 2034 Natl. Inst. Stand. Technol. Tech. Note 2034, 7 pages (February 2019) CODEN: NTNOEF This publication is available free of charge from: https://doi.org/10.6028/NIST.TN.2034 NIST Technical Note 2034 1 Defning ‘kind of quantity’ David Flater 2019-02-06 This publication is available free of charge from: https://doi.org/10.6028/NIST.TN.2034 Abstract The defnition of ‘kind of quantity’ given in the International Vocabulary of Metrology (VIM), 3rd edition, does not cover the historical meaning of the term as it is most commonly used in metrology. Most of its historical meaning has been merged into ‘quantity,’ which is polysemic across two layers of abstraction. -
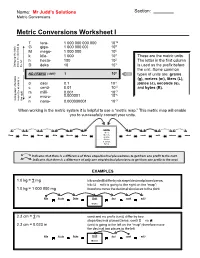
Metric Conversion Worksheet – Solutions
Name: Mr Judd’s Solutions Section: Metric Conversions Metric Conversions Worksheet I T tera- 1 000 000 000 000 1012 9 G giga- 1 000 000 000 10 M mega- 1 000 000 106 k kilo- 1 000 103 These are the metric units. h hecto- 100 102 The letter in the first column 1 Going table up the movethe decimal to theleft D deka 10 10 is used as the prefix before the unit. Some common NO PREFIX ( UNIT) 1 100 types of units are: grams (g), meters (m), liters (L), d deci 0.1 10-1 joules (J), seconds (s), c centi- 0.01 10-2 and bytes (B). m milli- 0.001 10-3 -6 μ micro- 0.000001 10 Goingtable the down movethe decimal to theright n nano- 0.000000001 10-9 When working in the metric system it is helpful to use a “metric map.” This metric map will enable you to successfully convert your units. Units Liters Tera Giga Mega Kilo Hecto Deka Meters deci centi milli micro nano Grams Joules Seconds Bytes indicates that there is a difference of three steps/decimal places/zeros to get from one prefix to the next. indicates that there is a difference of only one step/decimal place/zero to get from one prefix to the next. EXAMPLES 1.0 kg = ? mg 1.0 kg = 1 000 000 mg Kilo Hecto Deka Unit deci centi milli Grams 2.3 cm = ? m 2.3 cm = 0.023 m (unit) is going to the left on the “map”; therefore move the decimal two places to the left Kilo Hecto Deka Unit deci centi milli Meters From To Difference Move decimal in zeros/ left or right? prefix unit prefix unit decimal places/steps km kilo Meters m no prefix Meters 3 right Mg mega grams Gg giga grams 3 left mL milli Litres L no prefix Litres 3 left kJ kilo Joules mJ milli Joules 6 right μs micro seconds ms milli seconds 3 left B no prefix bytes kB kilo bytes 3 left cm centi metre mm milli metre 1 right cL centi Litres μL micro Litres 4 right PROBLEMS 1. -

Arithmetic Algorisms in Different Bases
Arithmetic Algorisms in Different Bases The Addition Algorithm Addition Algorithm - continued To add in any base – Step 1 14 Step 2 1 Arithmetic Algorithms in – Add the digits in the “ones ”column to • Add the digits in the “base” 14 + 32 5 find the number of “1s”. column. + 32 2 + 4 = 6 5 Different Bases • If the number is less than the base place – If the sum is less than the base 1 the number under the right hand column. 6 > 5 enter under the “base” column. • If the number is greater than or equal to – If the sum is greater than or equal 1+1+3 = 5 the base, express the number as a base 6 = 11 to the base express the sum as a Addition, Subtraction, 5 5 = 10 5 numeral. The first digit indicates the base numeral. Carry the first digit Multiplication and Division number of the base in the sum so carry Place 1 under to the “base squared” column and Carry the 1 five to the that digit to the “base” column and write 4 + 2 and carry place the second digit under the 52 column. the second digit under the right hand the 1 five to the “base” column. Place 0 under the column. “fives” column Step 3 “five” column • Repeat this process to the end. The sum is 101 5 Text Chapter 4 – Section 4 No in-class assignment problem In-class Assignment 20 - 1 Example of Addition The Subtraction Algorithm A Subtraction Problem 4 9 352 Find: 1 • 3 + 2 = 5 < 6 →no Subtraction in any base, b 35 2 • 2−4 can not do. -

Guide for the Use of the International System of Units (SI)
Guide for the Use of the International System of Units (SI) m kg s cd SI mol K A NIST Special Publication 811 2008 Edition Ambler Thompson and Barry N. Taylor NIST Special Publication 811 2008 Edition Guide for the Use of the International System of Units (SI) Ambler Thompson Technology Services and Barry N. Taylor Physics Laboratory National Institute of Standards and Technology Gaithersburg, MD 20899 (Supersedes NIST Special Publication 811, 1995 Edition, April 1995) March 2008 U.S. Department of Commerce Carlos M. Gutierrez, Secretary National Institute of Standards and Technology James M. Turner, Acting Director National Institute of Standards and Technology Special Publication 811, 2008 Edition (Supersedes NIST Special Publication 811, April 1995 Edition) Natl. Inst. Stand. Technol. Spec. Publ. 811, 2008 Ed., 85 pages (March 2008; 2nd printing November 2008) CODEN: NSPUE3 Note on 2nd printing: This 2nd printing dated November 2008 of NIST SP811 corrects a number of minor typographical errors present in the 1st printing dated March 2008. Guide for the Use of the International System of Units (SI) Preface The International System of Units, universally abbreviated SI (from the French Le Système International d’Unités), is the modern metric system of measurement. Long the dominant measurement system used in science, the SI is becoming the dominant measurement system used in international commerce. The Omnibus Trade and Competitiveness Act of August 1988 [Public Law (PL) 100-418] changed the name of the National Bureau of Standards (NBS) to the National Institute of Standards and Technology (NIST) and gave to NIST the added task of helping U.S. -

Multidisciplinary Design Project Engineering Dictionary Version 0.0.2
Multidisciplinary Design Project Engineering Dictionary Version 0.0.2 February 15, 2006 . DRAFT Cambridge-MIT Institute Multidisciplinary Design Project This Dictionary/Glossary of Engineering terms has been compiled to compliment the work developed as part of the Multi-disciplinary Design Project (MDP), which is a programme to develop teaching material and kits to aid the running of mechtronics projects in Universities and Schools. The project is being carried out with support from the Cambridge-MIT Institute undergraduate teaching programe. For more information about the project please visit the MDP website at http://www-mdp.eng.cam.ac.uk or contact Dr. Peter Long Prof. Alex Slocum Cambridge University Engineering Department Massachusetts Institute of Technology Trumpington Street, 77 Massachusetts Ave. Cambridge. Cambridge MA 02139-4307 CB2 1PZ. USA e-mail: [email protected] e-mail: [email protected] tel: +44 (0) 1223 332779 tel: +1 617 253 0012 For information about the CMI initiative please see Cambridge-MIT Institute website :- http://www.cambridge-mit.org CMI CMI, University of Cambridge Massachusetts Institute of Technology 10 Miller’s Yard, 77 Massachusetts Ave. Mill Lane, Cambridge MA 02139-4307 Cambridge. CB2 1RQ. USA tel: +44 (0) 1223 327207 tel. +1 617 253 7732 fax: +44 (0) 1223 765891 fax. +1 617 258 8539 . DRAFT 2 CMI-MDP Programme 1 Introduction This dictionary/glossary has not been developed as a definative work but as a useful reference book for engi- neering students to search when looking for the meaning of a word/phrase. It has been compiled from a number of existing glossaries together with a number of local additions. -
Calculus Terminology
AP Calculus BC Calculus Terminology Absolute Convergence Asymptote Continued Sum Absolute Maximum Average Rate of Change Continuous Function Absolute Minimum Average Value of a Function Continuously Differentiable Function Absolutely Convergent Axis of Rotation Converge Acceleration Boundary Value Problem Converge Absolutely Alternating Series Bounded Function Converge Conditionally Alternating Series Remainder Bounded Sequence Convergence Tests Alternating Series Test Bounds of Integration Convergent Sequence Analytic Methods Calculus Convergent Series Annulus Cartesian Form Critical Number Antiderivative of a Function Cavalieri’s Principle Critical Point Approximation by Differentials Center of Mass Formula Critical Value Arc Length of a Curve Centroid Curly d Area below a Curve Chain Rule Curve Area between Curves Comparison Test Curve Sketching Area of an Ellipse Concave Cusp Area of a Parabolic Segment Concave Down Cylindrical Shell Method Area under a Curve Concave Up Decreasing Function Area Using Parametric Equations Conditional Convergence Definite Integral Area Using Polar Coordinates Constant Term Definite Integral Rules Degenerate Divergent Series Function Operations Del Operator e Fundamental Theorem of Calculus Deleted Neighborhood Ellipsoid GLB Derivative End Behavior Global Maximum Derivative of a Power Series Essential Discontinuity Global Minimum Derivative Rules Explicit Differentiation Golden Spiral Difference Quotient Explicit Function Graphic Methods Differentiable Exponential Decay Greatest Lower Bound Differential -

Topic 0991 Electrochemical Units Electric Current the SI Base
Topic 0991 Electrochemical Units Electric Current The SI base electrical unit is the AMPERE which is that constant electric current which if maintained in two straight parallel conductors of infinite length and of negligible circular cross section and placed a metre apart in a vacuum would produce between these conductors a force equal to 2 x 10-7 newton per metre length. It is interesting to note that definition of the Ampere involves a derived SI unit, the newton. Except in certain specialised applications, electric currents of the order ‘amperes’ are rare. Starter motors in cars require for a short time a current of several amperes. When a current of one ampere passes through a wire about 6.2 x 1018 electrons pass a given point in one second [1,2]. The coulomb (symbol C) is the electric charge which passes through an electrical conductor when an electric current of one A flows for one second. Thus [C] = [A s] (a) Electric Potential In order to pass an electric current thorough an electrical conductor a difference in electric potential must exist across the electrical conductor. If the energy expended by a flow of one ampere for one second equals one Joule the electric potential difference across the electrical conductor is one volt [3]. Electrical Resistance and Conductance If the electric potential difference across an electrical conductor is one volt when the electrical current is one ampere, the electrical resistance is one ohm, symbol Ω [4]. The inverse of electrical resistance , the conductance, is measured using the unit siemens, symbol [S]. -

Output Watt Ampere Voltage Priority 8Ports 1600Max Load 10Charger
Active Modular Energy System 1600W Active Power and Control The processors on board the NCore Lite constantly monitor the operating status and all the parameters of voltage, absorption, temperature and battery charge status. The software operates proactively by analyzing the applied loads and intervening to ensure maximum operating life for the priority devices. All in 1 Rack Unit All the functionality is contained in a single rack unit. Hi Speed Switch All actuators and protection diodes have been replaced with low resistance mosfets ensuring instant switching times, efficiency and minimum heat dissi- pation. Dual Processor Ouput NCore Lite is equipped with 2 processors. One is dedicated to the monitoring ports and operation of the hardware part, the other is used for front-end manage- 8 ment. This division makes the system immune from attacks from outside. Watt typ load 1600 Port Status and Battery circuit breaker Ampere Load Indicator 10 charger Voltage OUT selectionable 800W AC/DC 54V Battery Connector Priority External thermistor system OPT 2 8 x OUTPUT ports Status & alarm indicator 9dot Smart solutions for new energy systems DCDC Input Module Hi Efficiency The DCDC Input module is an 800W high efficiency isolated converter with 54V output voltage. With an extended input voltage range between 36V and 75V, it can easily be powered by batteries, DC micro-grids or solar panels. Always on It can be combined with load sharing ACDC Input modules or used as the only power source. In case of overload or insufficient input, it goes into -

The Logarithmic Chicken Or the Exponential Egg: Which Comes First?
The Logarithmic Chicken or the Exponential Egg: Which Comes First? Marshall Ransom, Senior Lecturer, Department of Mathematical Sciences, Georgia Southern University Dr. Charles Garner, Mathematics Instructor, Department of Mathematics, Rockdale Magnet School Laurel Holmes, 2017 Graduate of Rockdale Magnet School, Current Student at University of Alabama Background: This article arose from conversations between the first two authors. In discussing the functions ln(x) and ex in introductory calculus, one of us made good use of the inverse function properties and the other had a desire to introduce the natural logarithm without the classic definition of same as an integral. It is important to introduce mathematical topics using a minimal number of definitions and postulates/axioms when results can be derived from existing definitions and postulates/axioms. These are two of the ideas motivating the article. Thus motivated, the authors compared manners with which to begin discussion of the natural logarithm and exponential functions in a calculus class. x A related issue is the use of an integral to define a function g in terms of an integral such as g()() x f t dt . c We believe that this is something that students should understand and be exposed to prior to more advanced x x sin(t ) 1 “surprises” such as Si(x ) dt . In particular, the fact that ln(x ) dt is extremely important. But t t 0 1 must that fact be introduced as a definition? Can the natural logarithm function arise in an introductory calculus x 1 course without the -

A HISTORICAL OVERVIEW of BASIC ELECTRICAL CONCEPTS for FIELD MEASUREMENT TECHNICIANS Part 1 – Basic Electrical Concepts
A HISTORICAL OVERVIEW OF BASIC ELECTRICAL CONCEPTS FOR FIELD MEASUREMENT TECHNICIANS Part 1 – Basic Electrical Concepts Gerry Pickens Atmos Energy 810 Crescent Centre Drive Franklin, TN 37067 The efficient operation and maintenance of electrical and metal. Later, he was able to cause muscular contraction electronic systems utilized in the natural gas industry is by touching the nerve with different metal probes without substantially determined by the technician’s skill in electrical charge. He concluded that the animal tissue applying the basic concepts of electrical circuitry. This contained an innate vital force, which he termed “animal paper will discuss the basic electrical laws, electrical electricity”. In fact, it was Volta’s disagreement with terms and control signals as they apply to natural gas Galvani’s theory of animal electricity that led Volta, in measurement systems. 1800, to build the voltaic pile to prove that electricity did not come from animal tissue but was generated by contact There are four basic electrical laws that will be discussed. of different metals in a moist environment. This process They are: is now known as a galvanic reaction. Ohm’s Law Recently there is a growing dispute over the invention of Kirchhoff’s Voltage Law the battery. It has been suggested that the Bagdad Kirchhoff’s Current Law Battery discovered in 1938 near Bagdad was the first Watts Law battery. The Bagdad battery may have been used by Persians over 2000 years ago for electroplating. To better understand these laws a clear knowledge of the electrical terms referred to by the laws is necessary. Voltage can be referred to as the amount of electrical These terms are: pressure in a circuit.