Ph: MEASURING ACIDITY and BASICITY the Activity
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Wastewater Technology Fact Sheet: Ammonia Stripping
United States Office of Water EPA 832-F-00-019 Environmental Protection Washington, D.C. September 2000 Agency Wastewater Technology Fact Sheet Ammonia Stripping DESCRIPTION Ammonia stripping is a simple desorption process used to lower the ammonia content of a wastewater stream. Some wastewaters contain large amounts of ammonia and/or nitrogen-containing compounds that may readily form ammonia. It is often easier and less expensive to remove nitrogen from wastewater in the form of ammonia than to convert it to nitrate-nitrogen before removing it (Culp et al., 1978). Ammonia (a weak base) reacts with water (a weak acid) to form ammonium hydroxide. In ammonia stripping, lime or caustic is added to the wastewater until the pH reaches 10.8 to 11.5 standard units which converts ammonium hydroxide ions to ammonia gas according to the following reaction(s): + - NH4 + OH 6 H2O + NH38 Source: Culp, et. al, 1978. Figure 1 illustrates two variations of ammonia FIGURE 1 TWO TYPES OF STRIPPING stripping towers, cross-flow and countercurrent. In TOWERS a cross-flow tower, the solvent gas (air) enters along the entire depth of fill and flows through the packing, as the alkaline wastewater flows it may be more economical to use alternate downward. A countercurrent tower draws air ammonia removal techniques, such as steam through openings at the bottom, as wastewater is stripping or biological methods. Air stripping may pumped to the top of a packed tower. Free also be used to remove many hydrophobic organic ammonia (NH3) is stripped from falling water molecules (Nutrient Control, 1983). droplets into the air stream, then discharged to the atmosphere. -

A Fiber Optic Ammonia Sensor Using a Universal Ph Indicator
Sensors 2014, 14, 4060-4073; doi:10.3390/s140304060 OPEN ACCESS sensors ISSN 1424-8220 www.mdpi.com/journal/sensors Article A Fiber Optic Ammonia Sensor Using a Universal pH Indicator Adolfo J. Rodríguez 1,*, Carlos R. Zamarreño 2, Ignacio R. Matías 2, Francisco. J. Arregui 2, Rene F. Domínguez Cruz 1 and Daniel. A. May-Arrioja 1 1 Fiber and Integrated Optics Laboratory, Electronics Engineering Department, UAM Reynosa Rodhe, Universidad Autónoma de Tamaulipas, Carr. Reynosa-San Fernando S/N, Reynosa, Tamaulipas 88779, Mexico; E-Mails: [email protected] (R.F.D.C.); [email protected] (D.A.M.-A.) 2 Departamento de Ingeniería Eléctrica y Electrónica, Universidad Pública de Navarra, Edif. Los Tejos, Campus Arrosadia, Pamplona España 31006, Spain; E-Mails: [email protected] (C.R.Z.); [email protected] (I.R.M.); [email protected] (F.J.A.) * Author to whom correspondence should be addressed; E-Mail: [email protected]; Tel.: +52-899-9213300 (ext. 8315); Fax: +52-899-921-3300 (ext. 8050). Received: 27 December 2013; in revised form: 12 February 2014 / Accepted: 18 February 2014 / Published: 27 February 2014 Abstract: A universal pH indicator is used to fabricate a fiber optic ammonia sensor. The advantage of this pH indicator is that it exhibits sensitivity to ammonia over a broad wavelength range. This provides a differential response, with a valley around 500 nm and a peak around 650 nm, which allows us to perform ratiometric measurements. The ratiometric measurements provide not only an enhanced signal, but can also eliminate any external disturbance due to humidity or temperature fluctuations. -

Properties of Acids and Bases
GREEN CHEMISTRY LABORATORY MANUAL Lab 22 Properties of Acids and Bases TN Standard 4.2: The student will investigate the characteristics of acids and bases. Have you ever brushed your teeth and then drank a glass of orange juice? hat do you taste when you brush your teeth and drink orange juice afterwards. Yuck! It leaves a really bad taste in your mouth, but why? Orange juice and toothpaste by themselves taste good. But the terrible taste W results because an acid/base reaction is going on in your mouth. Orange juice is a weak acid and the toothpaste is a weak base. When they are placed together they neutralize each other and produce a product that is unpleasant to taste. How do you determine what is an acid and what is a base? In this lab we will discover how to distinguish between acids and bases. Introduction Two very important classes of compounds are acids and bases. But what exactly makes them different? There are differences in definition, physical differences, and reaction differences. According to the Arrhenius definition, acids ionize in water to + produce a hydronium ion (H3O ), and bases dissociate in water to produce hydroxide ion (OH -). Physical differences can be detected by the senses, including taste and touch. Acids have a sour or tart taste and can produce a stinging sensation to broken skin. For example, if you have ever tasted a lemon, it can often result in a sour face. Bases have a bitter taste and a slippery feel. Soap and many cleaning products are bases. -

Multidisciplinary Design Project Engineering Dictionary Version 0.0.2
Multidisciplinary Design Project Engineering Dictionary Version 0.0.2 February 15, 2006 . DRAFT Cambridge-MIT Institute Multidisciplinary Design Project This Dictionary/Glossary of Engineering terms has been compiled to compliment the work developed as part of the Multi-disciplinary Design Project (MDP), which is a programme to develop teaching material and kits to aid the running of mechtronics projects in Universities and Schools. The project is being carried out with support from the Cambridge-MIT Institute undergraduate teaching programe. For more information about the project please visit the MDP website at http://www-mdp.eng.cam.ac.uk or contact Dr. Peter Long Prof. Alex Slocum Cambridge University Engineering Department Massachusetts Institute of Technology Trumpington Street, 77 Massachusetts Ave. Cambridge. Cambridge MA 02139-4307 CB2 1PZ. USA e-mail: [email protected] e-mail: [email protected] tel: +44 (0) 1223 332779 tel: +1 617 253 0012 For information about the CMI initiative please see Cambridge-MIT Institute website :- http://www.cambridge-mit.org CMI CMI, University of Cambridge Massachusetts Institute of Technology 10 Miller’s Yard, 77 Massachusetts Ave. Mill Lane, Cambridge MA 02139-4307 Cambridge. CB2 1RQ. USA tel: +44 (0) 1223 327207 tel. +1 617 253 7732 fax: +44 (0) 1223 765891 fax. +1 617 258 8539 . DRAFT 2 CMI-MDP Programme 1 Introduction This dictionary/glossary has not been developed as a definative work but as a useful reference book for engi- neering students to search when looking for the meaning of a word/phrase. It has been compiled from a number of existing glossaries together with a number of local additions. -

Drugs and Acid Dissociation Constants Ionisation of Drug Molecules Most Drugs Ionise in Aqueous Solution.1 They Are Weak Acids Or Weak Bases
Drugs and acid dissociation constants Ionisation of drug molecules Most drugs ionise in aqueous solution.1 They are weak acids or weak bases. Those that are weak acids ionise in water to give acidic solutions while those that are weak bases ionise to give basic solutions. Drug molecules that are weak acids Drug molecules that are weak bases where, HA = acid (the drug molecule) where, B = base (the drug molecule) H2O = base H2O = acid A− = conjugate base (the drug anion) OH− = conjugate base (the drug anion) + + H3O = conjugate acid BH = conjugate acid Acid dissociation constant, Ka For a drug molecule that is a weak acid The equilibrium constant for this ionisation is given by the equation + − where [H3O ], [A ], [HA] and [H2O] are the concentrations at equilibrium. In a dilute solution the concentration of water is to all intents and purposes constant. So the equation is simplified to: where Ka is the acid dissociation constant for the weak acid + + Also, H3O is often written simply as H and the equation for Ka is usually written as: Values for Ka are extremely small and, therefore, pKa values are given (similar to the reason pH is used rather than [H+]. The relationship between pKa and pH is given by the Henderson–Hasselbalch equation: or This relationship is important when determining pKa values from pH measurements. Base dissociation constant, Kb For a drug molecule that is a weak base: 1 Ionisation of drug molecules. 1 Following the same logic as for deriving Ka, base dissociation constant, Kb, is given by: and Ionisation of water Water ionises very slightly. -

Detergent Residue Testing Using a Ph Meter, Ph Indicator, Or Test Kit
Alconox, Inc. The Leader in Critical Cleaning 30 Glenn St. White Plains NY 10603 USA Tel 914.948.4040 Fax 914-948-4088 www.alconox.com [email protected] Detergent Residue Testing Using a pH Meter, pH indicator, or Test Kit The following test procedures are suitable for detecting detergent residues resulting from improper rinsing and can be used to meet laboratory accreditation guidelines and questionnaires such as the College of American Pathologist program of State water lab accreditation programs. A. pH Meter Method 1. Rinse a small clean beaker by filling and emptying 3 times with source water. 2. Fill a 4th time and measure pH using a pH meter. Record the pH as source water pH. 3. Using a piece of cleaned glassware you wish to test, fill about 10% full with source water (10ml into 100ml beaker). Use more water if necessary to get enough water to be able to sufficiently immerse the pH meter electrode in your measuring beaker. 4. Swish water in glassware to extract residues from all possible surfaces. 5. Take pH reading with pH meter and record as glassware pH. 6. Any significant increase in pH indicates possible alkaline detergent residue. A significant change is 0.2 or more pH units on a pH meter measuring to 0.1 pH units of sensitivity. A result of less than 0.2-pH units change indicates properly rinsed glassware. Note: If deionized water is used as the sample water, a slight amount (10-20 mg/L) of reagent grade, non-buffering salt (NaCl, CaCl2) should be added to the sample water to allow pH meter to function properly. -

Chem331 Lect 2 Water Ph Acid Base Buffer
Chapter 2 – Water and pH Water - one of the most important molecules in life. •70% of the bodies mass is water •2/3 of total body water is intracellular (55-66% body weight of men and 10% less for women) •The rest is interstitial fluid of which 25% is in the blood plasma. pH - The body tightly controls both the volume and pH of water. •The bicarbonate system is crucial for blood maintenance •changes of pH greater than 0.1 are dangerous and can lead to coma -diabetics Properties of water • Polarity • Hydrogen bonding potential Specific heat, heat of vaporization • It is the unique combination of properties of water • Nucleophilic that make it the perfect solvent for biological • Ionization systems. We will discuss each of these properties in • Water is an ideal more detail. biological solvent Water is close to a tetrahedral shape with the unshared electrons on the two sp3-hybridized orbital are in two corners and the hydrogen in others o Compared to a tetrahedron, CH4 (109 ) or NH3 the bond angle is smaller (109.5o and 107o vs.104.5o) Water has hydrogen bonding potential •H-bonds are non-covalent, weak interactions •H2O is both a Hydrogen donor and acceptor •One H2O can form up to four H-bonds What Are the Properties of Water? A comparison of ice and water, in terms of H-bonds and Motion • Ice: 4 H bonds per water molecule • Water: 2.3 H bonds per water molecule • Ice: H-bond lifetime - about 10 microsec • Water: H-bond lifetime - about 10 psec • (10 psec = 0.00000000001 sec) The Solvent Properties of Water Derive from Its Polar Nature •Water has a high dielectric constant •Ions are always hydrated in water and carry around a "hydration shell" •Water forms H bonds with polar solutes The Solvent Properties of Water Derive from Its Polar Nature What makes this molecule important? solvent ability - easily disrupts ionic compounds - dielectric constant (D) is high (measure of the ability to keep ions apart) – Large electronegativity creates a strong ionic type bond (dipole). -

Acid Base Chemistry Objectives Properties of Acids Properties Of
May 09, 2014 Objectives 1) Acid/Base Theories 2) pH scale and pH calculations 3) Measuring pH, pH scale Acid Base Chemistry 4) Strength of Acids and Bases Acid Base Chemistry -Equilibrium (Honors) Chapter 19 Properties of Acids Three Acid and Base Theories • Aqueous solutions of acids taste sour. Change the color of acid/base indicators • 1. Arrhenius Theory • Many will conduct electrical currents. Acid Rxns with Metals 1. Al, Mg and Zn form hydrogen gas 2. Bronsted-Lowry Theory 2Al + 6HCl 2AlCl3 + 3H2 2. Metal carbonates form carbon dioxide 3. Lewis Theory CaCO3 + 2HCl CaCl2 + H2O + CO2 Properties of Bases • Aqueous solutions have a bitter taste. • Change the color of acid/base indicators • Many react with acids to form salts in water. • They often feel slimy to the touch. • Solutions that are basic are often called alkaline. Examples of Arrhenius 1) Arrhenius Theory An acid in water ionizing to form a H+ ion Svante Arrhenius published a paper + - about how acids, bases, and salts can HCl(aq) H (aq) + Cl (aq) conduct an electric current. Concluded that both acids and bases A base in water dissociating to form hydroxide ion, OH- must release a charged particle in solution. Acids ionized to produce H+ and + - Bases ionized to produce hydroxide NaOH Na (aq) + OH (aq) ions OH- May 09, 2014 2) Bronsted-Lowry Theory Bronsted-Lowry Theory: General acid/base reaction This definition is an expansion on the Arrhenius acid definition. Acid + Base Conjugate base + Conjugate Acid In a chemical reaction, any substance that donates a proton is an acid and any substance that accepts a Conjugate base: particle that remains after a proton proton is a base. -
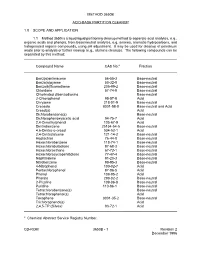
Method 3650B: Acid-Base Partition Cleanup, Part of Test Methods For
METHOD 3650B ACID-BASE PARTITION CLEANUP 1.0 SCOPE AND APPLICATION 1.1 Method 3650 is a liquid-liquid partitioning cleanup method to separate acid analytes, e.g., organic acids and phenols, from base/neutral analytes, e.g. amines, aromatic hydrocarbons, and halogenated organic compounds, using pH adjustment. It may be used for cleanup of petroleum waste prior to analysis or further cleanup (e.g., alumina cleanup). The following compounds can be separated by this method: _______________________________________________________________________________ Compound Name CAS No.a Fraction Benz(a)anthracene 56-55-3 Base-neutral Benzo(a)pyrene 50-32-8 Base-neutral Benzo(b)fluoranthene 205-99-2 Base-neutral Chlordane 57-74-9 Base-neutral Chlorinated dibenzodioxins Base-neutral 2-Chlorophenol 95-57-8 Acid Chrysene 218-01-9 Base-neutral Creosote 8001-58-9 Base-neutral and Acid Cresol(s) Acid Dichlorobenzene(s) Base-neutral Dichlorophenoxyacetic acid 94-75-7 Acid 2,4-Dimethylphenol 105-67-9 Acid Dinitrobenzene 25154-54-5 Base-neutral 4,6-Dinitro-o-cresol 534-52-1 Acid 2,4-Dinitrotoluene 121-14-2 Base-neutral Heptachlor 76-44-8 Base-neutral Hexachlorobenzene 118-74-1 Base-neutral Hexachlorobutadiene 87-68-3 Base-neutral Hexachloroethane 67-72-1 Base-neutral Hexachlorocyclopentadiene 77-47-4 Base-neutral Naphthalene 91-20-3 Base-neutral Nitrobenzene 98-95-3 Base-neutral 4-Nitrophenol 100-02-7 Acid Pentachlorophenol 87-86-5 Acid Phenol 108-95-2 Acid Phorate 298-02-2 Base-neutral 2-Picoline 109-06-8 Base-neutral Pyridine 110-86-1 Base-neutral Tetrachlorobenzene(s) Base-neutral Tetrachlorophenol(s) Acid Toxaphene 8001-35-2 Base-neutral Trichlorophenol(s) Acid 2,4,5-TP (Silvex) 93-72-1 Acid _______________________________________________________________________________ a Chemical Abstract Service Registry Number. -

Acids and Bases Experiments (Organic Classes)
Acids and Bases Experiments (Organic Classes) NaHCO3 (sodium bicarbonate, baking soda) and vinegar (dilute acetic acid) will be used to shoot a cork out of a bottle to show that some acid-base reactions generate gases like CO2. All students in the class will participate in a voice-activated chemical reaction. Students will remove a stopper and speak into a flask containing base and an indicator that will change color once enough CO2 is introduced from the students’ breath. Further color changes from basic to acidic conditions are illustrated using natural and household liquids and a universal indicator prepared from red cabbage juice. Finally, conversion between colorless and colored forms of different acid-base indicators in unusual settings will be shown in the “invisible painting” experiment. Supplies Needed: For the rocket reaction: You provide the following items indicated in red and starred: * empty 1 L plastic soda bottle with label removed * vinegar (colorless) Plastic powder funnel (we provide) cork with streamers attached by a thumbtack (we provide) container of de-ionized water (we provide, you refill as needed) sodium bicarbonate (we provide, return unused) Please clean and return all materials For the voice-activated chemical reaction: water that we provide as 250 mL Erlenmeyer flask (we provide)) soon as possible. dropper bottle with colorless phenolphtalein indicator (we provide) Other students may small amount of 1 M NaOH solution (we provide) need to use this “kit” disposable plastic droppers (we provide) for their ChemDemo!! For the universal pH indicator from red cabbage: * Red cabbage juice (you make the day before the demo) * 1 lemon, 1 soap bar, Sprite, vinegar, drain cleaner * 7 stirring rods (e.g. -

Estimating Ph at the Air/Water Interface with a Confocal
ANALYTICAL SCIENCES OCTOBER 2015, VOL. 31 1 2015 © The Japan Society for Analytical Chemistry Supporting Information Estimating pH at the Air/Water Interface with a Confocal Fluorescence Microscope Haiya YANG, Yasushi IMANISHI, Akira HARATA† Department of Molecular and Material Sciences, Interdisciplinary Graduate School of Engineering Sciences, Kyushu University, 6-1 Kasugakoen, Kasuga-shi, Fukuoka 816-8580, Japan † To whom correspondence should be addressed. Email: [email protected] 1 2 ANALYTICAL SCIENCES OCTOBER 2015, VOL. 31 Mathematical relationship between fluorescence peak wavenumbers and pH for a fluorescent pH indicator In a confocal fluorescence microscope, the probe volume is confined in an elongated cylindrical shape with radius and height . If the position of the surface is defined to be exactly at the symmetrical plane horizontally intersecting the cylinder, the probe area and probe volume are and for the surface observation, while they are zero and for the bulk observation, respectively. For a component i, the ratio of fluorescent intensity detected for the surface observation with respect to the bulk observation can be given as (S1) where and represent the efficiencies of fluorescence excitation detection per fluorescent molecule at the surface and in the bulk solution, respectively; is the surface density, and is the bulk concentration. Because , Eq. (S1) is deformed into ; (S2) when , a surface-selective observation for this surface-active component at the water surface is available.17 In this case, and at a low concentration limit, the pH-dependent fluorescence spectrum of the surface-adsorbed pH indictor is given by , (S3) 2 ANALYTICAL SCIENCES OCTOBER 2015, VOL. -

Ph (Colorimetric) See a Separate Application for the Arena Or Gallery Analyzer
Page 1 D10493_03_Insert_pH_Colorimetric EN TEST PROCEDURE pH (colorimetric) See a separate application for the Arena or Gallery analyzer. 984349 Materials required but not provided 3 x 20 ml Reagent 1 Distilled water (aseptic and free of heavy metals) and general laboratory equipment. INTENDED USE Reagent for photometric determination of pH (colorimetric) in Calibrators (pH 3, 4, 5, 6). homogenous liquid samples using automated Thermo Scientific Arena pH 4 Std for QC, Thermo Fisher Scientific Cat no 984331 or Gallery analyzer. pH 7 Std for QC, Thermo Fisher Scientific Cat no 984332 METHOD Calibration Colorimetric test with pH indicator dyes in an aqueous solution. This method has been developed using 4 separate calibration points. Method is performed at 37 °C, using 575 nm filter and 700 nm as side Calibrators used were: wavelenght. Fixanal pH 3, Fixanal pH 4, Fixanal pH 5 and Fixanal pH 6 from PRINCIPLE OF THE PROCEDURE Sigma-Aldrich. pH (colorimetric) method is based on the property of acid-base indicator dyes, which produce color depending on the pH of the Note: Samples measured with manual pH meter can also be used as sample. The color change can be measured as an absorbance calibrators. In this case the calibration must be performed with same change spectrophotometrically. matrix type and with several points covering the whole measuring range. This calibration type must be validated by the user. REAGENT INFORMATION Barcode ID Quality Control Reagent 1 (R1) 3 x 20 ml A13 Use quality control samples at least once a day and after each calibration and every time a new bottle of reagent is used.