Assessing Coral Reef Health in the North Ari Atoll (Maldives) Using the Foram Index
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Population and Housing Census 2014
MALDIVES POPULATION AND HOUSING CENSUS 2014 National Bureau of Statistics Ministry of Finance and Treasury Male’, Maldives 4 Population & Households: CENSUS 2014 © National Bureau of Statistics, 2015 Maldives - Population and Housing Census 2014 All rights of this work are reserved. No part may be printed or published without prior written permission from the publisher. Short excerpts from the publication may be reproduced for the purpose of research or review provided due acknowledgment is made. Published by: National Bureau of Statistics Ministry of Finance and Treasury Male’ 20379 Republic of Maldives Tel: 334 9 200 / 33 9 473 / 334 9 474 Fax: 332 7 351 e-mail: [email protected] www.statisticsmaldives.gov.mv Cover and Layout design by: Aminath Mushfiqa Ibrahim Cover Photo Credits: UNFPA MALDIVES Printed by: National Bureau of Statistics Male’, Republic of Maldives National Bureau of Statistics 5 FOREWORD The Population and Housing Census of Maldives is the largest national statistical exercise and provide the most comprehensive source of information on population and households. Maldives has been conducting censuses since 1911 with the first modern census conducted in 1977. Censuses were conducted every five years since between 1985 and 2000. The 2005 census was delayed to 2006 due to tsunami of 2004, leaving a gap of 8 years between the last two censuses. The 2014 marks the 29th census conducted in the Maldives. Census provides a benchmark data for all demographic, economic and social statistics in the country to the smallest geographic level. Such information is vital for planning and evidence based decision-making. Census also provides a rich source of data for monitoring national and international development goals and initiatives. -

National Geospatial Database for Maldives to Mainstream Climate Change Adaptation in Development Planning (ADB Brief No. 117)
NO. 117 November 2019 ADB BRIEFS KEY POINTS National Geospatial Database for • The Republic of Maldives is one of the most biodiverse Maldives to Mainstream Climate countries in the world, yet it is among the most vulnerable Change Adaptation in Development to climate change. The country needs to ensure the Planning sustainable management of natural resources in spite of the impacts and consequences of climate Liping Zheng change. Advisor • The government’s Asian Development Bank environmental management and resource conservation National Consultant Team: efforts that began in the early 1990s have been constrained Ahmed Jameel Hussain Naeem by a lack of relevant data and Integrated Coastal Zone Coastal Ecosystems and Biodiversity information. Management Specialist Specialist • This brief presents the Faruhath Jameel Mahmood Riyaz development of a geospatial Geographic Information Systems database and maps to help Climate Change Risk Assessment Maldives (i) assess disaster Specialist and Team Leader Specialist risks and impacts; (ii) reduce these by strengthening the design of programs and policies; and (iii) mainstream BACKGROUND climate change adaptation in development planning. Maldives is a developing state composed of 26 natural atolls with about 1,192 small coral islands spread over roughly 90,000 square kilometers in the Indian Ocean. The country • A geospatial database is divided into 20 administrative regions, each with a local administrative authority on coastal and marine governed by the central government. With some of the world’s most beautiful beaches, ecosystems that includes Maldives has relied on high-end tourism to expand its economy over recent decades climate risk assessment and gained middle-income status with the highest per capita income in South Asia.1 information makes it feasible to screen for climate risks in Maldives is characterized by extremely low elevations and, as one of the most development projects and geographically dispersed countries in the world, it is among the most vulnerable to programs at national and climate change. -

Villas and Residences | Club Intercontinental Benefits | Opening Special | Getting Here
VILLAS AND RESIDENCES | CLUB INTERCONTINENTAL BENEFITS | OPENING SPECIAL | GETTING HERE RESTAURANTS AND BARS | OCEAN CONSERVATION PROGRAM & COLLABORATION WITH MANTA TRUST | AVI SPA & WELLNESS AND KIDS CLUB A new experience lies ahead of you this September with the opening of the new InterContinental Maldives Maamunagau Resort. Spread over a private island with lush tropical greenery, InterContinental Maldives Maamunagau Resort seamlessly blends with the awe-inspiring natural beauty of the island. Resort facilities include: • 81 Villas & Residences • 6 restaurants and bars • Club InterContinental benefits • “The Retreat” - an adults only lounge • An overwater spa • 5 Star PADI certified diver center oering courses and daily expeditions with an on-site Marine Biologist • Planet Trekkers children’s facility VILLAS AND RESIDENCES Experience Maldives’ breathtaking vistas from each of the spacious 81 Beach, Lagoon and Overwater Villas and Residences at the InterContinental Maldives Maamunagau Resort. Choose soothing lagoon or dramatic ocean views with a perfect vantage point from your private terrace for a spectacular Bedroom - Overwater Pool Villa Outdoor Pool Deck - Overwater Pool Villa sunrise or sunset. Each Villa or Residence is tastefully designed encapsulating the needs of the modern nomad infused with distinct Maldivian design; featuring one, two or three separate bedrooms, lounge with an ensuite complemented by a spacious terrace overlooking the ocean or lagoon with a private pool. GO TO TOP Livingroom - Lagoon Pool Villa Bedroom - One -

Base Information Malé, Hulhumalé, Maldives
Base information Malé, Hulhumalé, Maldives We make your most important time of the year to your most beautiful experience. 1 yachts yachts supermarket supermarket Useful information airport Transfer After arrival by plane we pick you up and bring you to the yacht. Please let us know your arrival time. The costs for the transfer are already covered with the comfort package. Address BLUE HORIZON Pte Ltd M.Bolissafaru, 2nd Floor, Orchid Magu, Malé, Maldives GPS: 4.177213, 73.506887 Supermarket Our office can be found in the north of Malé (see map). The yachts are about Near the yachts is a large supermarket 20 minutes away, at Hulhumalé. (Redwave City Square). The two islands are connected by a GPS: 4.211042, 73.542010 bridge and can be reached by taxi or shuttle. Opening hours: daily 09:00 – 18:00 h Contact persons: 20:00 – 22:00 h Base manager: The supermarket can be reached by Mr. Ahmed Zubair Adam taxi, which we gladly organize for 00960 77 88 425 you. The taxi costs about € 5. Office: Mr. Ameer Abbas (00960 794 11 69) Mrs. Lorna (00960 795 11 62) Errors and mistakes reserved. 2 What to do in case of damage? Please contact the base immediately! Exchange insurance policy data (for liability damage) Take pictures of the damage Create a sketch with description of how the accident happened and let Damages can happen even to very experi- it sign from all involved persons enced skippers. Please let us know straight away when damage occurs, so we can Create a record with the port organise everything and so you don’t lose captain valuable holiday time. -

Introduction
THE JEWEL ISLAND. Ihavandhippolhu Atoll INTRODUCTION North Thiladhunmathee Atoll (Haa Alifu) South Thiladhunmathee Atoll Maamakunudhoo Atoll (Haa Dhaalu) North Miladhunmadulu Atoll (Shaviyani) North Maalhosmadulu Atoll (Raa) South Miladhunmadulu Atoll CONSTANCE MOOFUSHI MALDIVES (Noonu) Constance Moofushi Maldives is situated on the South Ari South Maalhosmadulu Atoll Faaddhippolhu Atoll (Baa) (Lhaviyani) Atoll and is widely regarded as one of the best diving spots in the world. The Resort combines the Crusoe Chic Barefoot Goidhoo Atoll Malé Atoll elegance of a deluxe resort with the highest standards of Rasdhoo Atoll Ari Atoll Malé Constance Hotels and Resorts. (Alifu) South Malé Atoll Moofushi Felidhoo Atoll (Vaavu) North Nilandhoo Atoll (Faafu) Vattaru Falhu Mulaku Atoll South Nilandhoo Atoll (Meemu) (Dhaalu) Kolhumadulu Atoll (Thaa) MALDIVES South Hadhdhunmathee Atoll Ari Atoll (Laamu) MOOFUSHI North Huvadhoo Atoll (Gaafu Alifu) South Huvadhoo Atoll (Gaafu Dhaalu) Foammulah Atoll (Gnaviyani) Addu Atoll (Seenu) VILLA’S FACILITIES All Beach and Water Villas feature air-conditioning, ceiling fan, bathroom, shower, WC, hairdryer, sitting area, complimentary WIFI, LCD TV, mac mini (iPod connection, CD & DVD), telephone, mini-bar, safe, tea, coffee facilities and a wooden terrace. All Senior Water Villas feature air-conditioning, ceiling fan, bathroom with outdoor bath tub, double vanities, shower, WC, hairdryer, sitting area, complimentary WIFI, LCD TV, mac mini (iPod connection, CD & DVD), telephone, mini-bar, safe, tea, coffee facilities and wooden terrace. ACCOMMODATION 24 BEACH VILLAS - (57 m2) 2 adults + 1 extra bed (adult or child under 12 years) 56 WATER VILLAS - (66 m2) 2 adults + 1 extra bed (adult or child under 12 years) SENIOR WATER VILLAS - (94 m2) 2 adults + 1 extra bed adult or 2 extra beds for children under 12 years RESTAURANT & BAR Constance Moofushi Maldives has 2 restaurants and 2 bars and guests enjoy the “Cristal” all-inclusive package during their stay. -

Private Health Facilities Registered Under Ministry of Health Tuesday, March 06, 2018
Ministry of Health Male, Republic of Maldives Private Health Facilities Registered under Ministry of Health Tuesday, March 06, 2018 S/no Reg No. Catagory Clinic Name Clinic Address Clinic Phone No Registered Date 1 HF-10/ALP/PVT/006 Medical Clinic Azmi Naeem Medical & Diagnostic Centre M. Misruvaage, Shaariuvarudhee Magu, Male' 3325979 09.10.1991 2 HF-10/ALP/PVT/007 Hospital ADK Hospital Sosun Magu, Male' 3313553 15.03.1996 3 HF-10/ALP/PVT/009 Medical Clinic The Clinic H. Shady Palm, Hithafinivaa magu, Male' 3323207 18.09.1994 4 HF-10/ALP/PVT/012 Medical Clinic Crescent Medical Services G. Hithifaru, Majeedhee Magu, Male' 3317411 14.09.1997 5 HF-10/ALP/PVT/013 Medical Clinic Poly Clinic Ma. Ran Ihi, Chaandhany Magu, Male' 3314647 14.07.1997 6 HF-10/ALP/PVT/014 Dental Clinic Dental Care Centre Ma. Meyvaagasdhoshuge (1st floor), Joashee Hingun, Male' 3328446 02.07.1998 7 HF-10/ALP/PVT/016 Medical Clinic Family Planning Centre Kulunu Vehi, Buruzu Magu, Male' 26.10.1998 8 HF-10/ALP/PVT/019 Medical Clinic Imperial Medical Centre G. Chaman, Lonuziyaarai Magu, Male' 33,166,003,336,887 13.10.1999 9 HF-10/ALP/PVT/021 Medical Clinic Royal Island Medical Centre Royal Island Resort, Baa Atoll 6600088, 6600099 12.06.2001 10 HF-10/ALP/PVT/023 Medical Clinic Kulhudhuffushi Medical Centre Fithuroanuge, Hdh. Kulhudhuffushi 08.01.2002 11 HF-10/ALP/PVT/025 Medical Clinic Central Clinic Ma. Dhilleevilla, Majeedhee Magu, Male' 3312221, 3328710, 10.06.2002 12 HF-10/ALP/PVT/026 Dental Clinic Smiles Dental Care Ma. -

Response of Tidal Flow Regime and Sediment Transport in North Male
https://doi.org/10.5194/os-2020-80 Preprint. Discussion started: 7 September 2020 c Author(s) 2020. CC BY 4.0 License. Response of tidal flow regime and sediment transport in North Male’ Atoll, Maldives to coastal modification and sea level rise Shuaib Rasheed 1, Simon C. Warder 1, Yves Plancherel 1,2, and Matthew D. Piggott 1 1Department of Earth Science and Engineering, Imperial College London, UK 2Grantham Institute – Climate Change and the Environment, Imperial College London, UK Correspondence: Shuaib Rasheed ([email protected]) Abstract. Changes to coastlines and bathymetry alter tidal dynamics and associated sediment transport process, impacting upon a number of threats facing coastal regions, including flood risk and erosion. Especially vulnerable are coral atolls such as those that make up the Maldives archipelago which has undergone significant land reclamation in recent years and decades, and is also particularly exposed to sea level rise. Here we develop a tidal model of Male’ Atoll, Maldives, and use it to assess 5 potential changes to sediment grain size distributions under sea level rise and coastline alteration scenarios. The results indicate that the impact of coastline modification over the last two decades at the island scale is not limited to the immediate vicinity of the modified island, but can also significantly impact the sediment grain size distribution across the wider atoll basin. Additionally, the degree of change in sediment distribution which can be associated with sea level rise that is projected to occur over relatively long time periods is predicted to occur over far shorter time periods with coastline changes, highlighting the 10 need to better understand, predict and mitigate the impact of land reclamation and other coastal modifications before conducting such activities. -
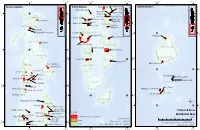
Protected Areas Distribution
73°0'0"E 74°0'0"E 73°0'0"E 74°0'0"E 73°0'0"E 74°0'0"E Northern Maldives Central Maldives Rasfari beyru Huraa Mangrove Area Southern Maldives Laamu Atoll Rasdhoo Madivaru Girifushi Thila Banana Reef Nassimo Thila 7°0'0"N 7°0'0"N Kuda Haa Lions Head Hans Hass Place; HP Reef Haa Alifu Atoll Mayaa Thila &% Kari beyru Thila Baarah Kulhi Emboodhoo Alifu Alifu Atoll Kanduolhi Orimas Thila 4°0'0"N Kaafu Atoll 4°0'0"N Haa Dhaalu Atoll Fish Head Guraidhoo &% Kanduolhi &% Keylakunu Neykurendhoo Mangrove Hurasdhoo Alifu Dhaalu Atoll 1°0'0"N 1°0'0"N Kudarah Thila Hithaadhoo Rangali Kandu Dhevana Kandu Shaviyani Atoll &% Farukolhu South Ari Atoll MPA Vaavu Atoll Filitheyo Kandu Gaafu Alifu Atoll Vattaru Kandu 6°0'0"N 6°0'0"N Faafu Atoll Noonu Atoll Gaafu Dhaalu Atoll Fushee Kandu Meemu Atoll 3°0'0"N Hakuraa Thila 3°0'0"N Kuredu Express Dhigulaabadhoo Raa Atoll &% Dhaalu Atoll &% Fushivaru Thila 0°0'0" 0°0'0" &% Bathala Region Anemone City &% Lhaviyani Atoll Mendhoo Region Angafaru Thoondi Area Dhandimagu Kilhi &% Maahuruvalhi &% &% &% &% Hanifaru Bandaara Kilhi Thaa Atoll Gnaviyani Atoll Baa Atoll Dhigali Haa &% 5°0'0"N Olhugiri 5°0'0"N Kan'di hera The Wreck of Corbin&% &% Hithadhoo Protected Area Goidhoo Koaru &% Seenu Atoll Mathifaru Huraa British Loyalty 2°0'0"N 2°0'0"N Laamu Atoll Makunudhoo channel &% Kaafu Atoll ¶ Rasfari beyru&% Huraa Mangrove Area 1°0'0"S 1°0'0"S &% Rasdhoo Madivaru &% Girifushi Thila &% Protected Areas &% Nassimo Thila &% Legend Kuda Haa &%Male' CityBanana Reef Kari beyru Thila &% &% Distribution Map Mayaa Thila Lions Head Hans Hass Place Protected Areas 2019 (Total 50 sites) 0 25 50 100 Km &% &% &% Sources: EPA 2019 Alifu Alifu Atoll Emboodhoo Islands Kanduolhi Map version Date: 30/06/2019 &% Orimas Thila Projection: Transverse Mercator (UTM Zone 43 N); 4°0'0"N &% 4°0'0"N Reefs Prepared by: Ministry of Environment, Maldives Fish Head &%Guraidhoo Kanduolhi Horizontal Datum: WGS84; 73°0'0"E 74°0'0"E 73°0'0"E 74°0'0"E 73°0'0"E 74°0'0"E. -

Of Whale Sharks (Rhincodon Typus) in South Ari Atoll and Baa Atoll, Maldives
Summer Placement Dissertation Motivations behind the ‘Site Fidelity’ of whale sharks (Rhincodon typus) in South Ari Atoll and Baa Atoll, Maldives Samuel Dixon Y0076632 MSc Marine Environmental Management Supervisors: Richard Rees, James Hancock and Guy Stevens Disclaimer: I confirm that all the work presented is my own and was carried out in the manner described below. My word count is 4927 words, discounting the title page, the disclaimer, my acknowledgments, my bibliography, both my appendices and all figures, tables, graphs and their legends. Acknowledgments I would like to thank both the Maldives Whale Shark Research Programme and the Manta Trust for all their help in facilitating this research. In particular to Richard Rees, James Hancock and Guy Stevens for all the advice, support and guidance they have given, both when I was in the field and back in york. A huge thank-you both the MWSRP’s amazing infield co-ordinators Alexandra Childs and Irthisham Zareer for all their patience, help and support during my time spent with them in the Maldives. I would like to thank Niv Froman for all the help and information he provided regarding mantas and Baa Atoll. I would also like to thank to the Dhoni crew at Dhigurah, without whom this project would not have been possible. I would further like to extend my thanks to all the stakeholders of the Big Fish Network for all the data and research they have contributed over the years. Thank-you too to all the MWSRP’s volunteers who all made my time in the Maldives so enjoyable. -

Study on Post-Tsunami Restoration and Conservation Projects in the Maldives
Study on Post-Tsunami Restoration and Conservation Projects in the Maldives Ecosystems and Livelihoods Group, Asia Study on Post-Tsunami Restoration and Conservation Projects in the Maldives Marie Saleem and Shahaama A. Sattar February 2009. Cover photo: Thaa Vilufushi after reclamation © Hissan Hassan Table of Contents 1 Introduction ................................................................................................... 3 2 Summary of post-tsunami restoration and conservation initiatives ............... 7 3 ARC/CRC Waste Management Programme .............................................. 11 3.1 Background ......................................................................................... 11 3.2 Summaries of outcomes in the Atolls .................................................. 12 3.2.1 Ari Atoll ......................................................................................... 13 3.2.2 Baa Atoll ....................................................................................... 13 3.2.3 Dhaalu Atoll .................................................................................. 13 3.2.4 Gaaf Alifu and Gaaf Dhaalu Atolls ................................................ 14 3.2.5 Haa Alifu Atoll............................................................................... 14 3.2.6 Haa Dhaalu Atoll .......................................................................... 15 3.2.7 Kaafu and Vaavu Atolls ................................................................ 15 3.2.8 Laamu Atoll ................................................................................. -

ITINERARIES LIST and DESCRIPTION Please, Note, Below
ITINERARIES LIST AND DESCRIPTION Please, note, below itineraries are samples. The actual route will depend on the current weather conditions and overall diver levels. The boat manager reserves the right to change/amend the itinerary to a more appropriate one, to provide best possible diving experience at the time of the cruise. Best Of Maldives, 7 nights, start and finish points in Male 1st day Arrivals, Briefings & Set Up. No diving. 2nd Day Check Dive North Male – Kurumba House Reef/ Lankan Manta Point Dive South Male - Emboodhoo Express (Sharks & Eagle Rays) Dive South Male - Kuda Giri Wreck 3rd Day Dive South Male - Kandhooma Thila (Sharks & Eagle Rays) Dive South Male - Guraidhoo Corner or Medu Faru (Sharks & Eagle Rays) Cross to Ari Atoll (4.5Hrs) 4th Day Dive South Ari Atoll - Kuda Rah Thila Look for Whalesharks - Maamagili Beyru Dive South Ari Atoll - Maamagili Beyru (Whalesharks) Dive South Ari Atoll - Rangali Madivaru (Manta Point) Night Fishing upon request 5th Day Dive South Ari Atoll - Radhiga Thila (Sharks + Great Coral) Dive North Ari Atoll - Dhonkalo Thila (Manta Point) Dive North Ari Atoll - Fish Head Desert Island Dinner 6th Day Dive North Ari Atoll - Maalhos Thila Dive North Ari Atoll - Hafsa Thila Dive North Ari Atoll - Maaya Thila Dive North Ari Atoll - Maaya Thila (Night Dive) 7th Day Dive North Ari Atoll - Reethi Thila Dive North Ari Atoll - Bathala Kan Thila Cross to Male Shopping and City Tour Male 8th Day Check Out 7&9am Pelagic Magic, 7 nights, start and finish points in Male North Ari Atoll: Grey Reef Sharks, Maaya Thila Night Dive, Multiple Manta Cleaning Stations South Ari Atoll: W halesharks Beautiful Pinnacles. -

Russian Factsheet 2019
Курорт вашей мечты Наш морской курорт предлагает 160 просторных современных вилл, расположенных на воде, на пляже или в саду. Из каждой виллы открываются великолепные виды белых песчаных пляжей и сверкающих океанских просторов. Вас ждут щедрое солнце и масса развлечений как для любителей приключений, так и для тех, кто хочет достичь полной релаксации - от захватывающего дух полета по гигантским волнам до расслабляющих спа-процедур в салоне Kandooma Spa by COMO Shambhala. А пока родители открывают для себя сокровища морских глубин под присмотром инструкторов 5-звездочного дайвинг-центра PADI, наши маленькие гости могут целый день играть в клубе для детей Kandoo Kids Club. Огромный выбор блюд международной и местной кухни и отменное качество еды превращают каждый прием пищи в настоящий пир. Мы делаем все, чтобы дни, проведенные в курортном отеле Holiday Inn Resort Kandooma Maldives оставили у наших гостей самые приятные воспоминания. Holiday Inn Resort Kandooma Maldives Kandooma Fushi, South Male Atoll, Republic of Maldives T: +960 664 511 | F: +960 664 0513 | maldives.holidayinnresorts.com Свадьбы Месторасположение HAA ALIFU ATOLL Хотите отпраздновать рождение своей семьи только вдвоем или Курорт Holiday Inn Resort Kandooma Maldives находится в атолле устроить грандиозный прием для родных и друзей? Наши HAA DHAALU ATOLL SHAVIYANI ATOLL Южный Мале в 35 км к югу от города Мале. Быстроходный катер свадебные специалисты позаботятся о том, чтобы свадьба стала NOONU ATOLL всего за 45 минут домчит вас сюда из международного аэропорта для вас ярким запоминающимся событием. Пакеты Renewal of RAA ATOLL Велана. После прохождения паспортного и таможенного контроля LHAVIYANI ATOLL Vows (возобновление свадебных обещаний) или Tropical Wedding наши представители встретят вас в эропорту и проводят в зал BAA ATOLL Ceremony (тропическая свадьба) включают массу таких NORTH MALE ATOLL ожидания, где вы сможете отдохнуть до отправления катера.