A Choosing Wisely Literature Synopsis
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Opioid Prescribing and Pain Management
Opioid Prescribing and Pain Management: Prescription Monitoring Program Overview and the Management of Acute Low Back Pain Conflict of Interest Disclosure and Funding Support Content Creation: CADTH Disclosures CADTH is an independent, not-for-profit organization funded by Canada’s federal, provincial, and territorial governments, with the exception of Quebec CADTH receives application fees for three programs: • CADTH Common Drug Review (CDR) • CADTH pan-Canadian Oncology Drug Review (pCODR) • CADTH Scientific Advice Relationships with commercial interests: None CADTH Opioid Prescribing and Acute Low Back Pain Module Collaborators Special acknowledgement and appreciation to the following organizations who supported the development of this slide deck: • New Brunswick Department of Health • New Brunswick Medical Society o Choosing Wisely New Brunswick CADTH Opioid Prescribing and Acute Low Back Pain Module Learning Objectives • Describe the risks associated with opioid use (including overdose, duration of therapy, and drug combinations). • Review the objectives of the prescription monitoring program (PMP) and how it can support decision-making at the point of care. • Examine the appropriate management of acute low back pain in the primary care setting. • Identify strategies for communicating the risks versus benefits of opioid therapy with patients. CADTH Opioid Prescribing and Acute Low Back Pain Module “The roots of what we now call the opioid crisis can be traced back many years to the promotion of opioid prescribing as low-risk, non-addictive, -

Ten Things Physicians and Patients Should Question 3 1 2
The American College of Obstetricians and Gynecologists Ten Things Physicians and Patients Should Question Don’t schedule elective, non-medically indicated inductions of labor or Cesarean deliveries before 39 weeks 0 days gestational age. 1 Delivery prior to 39 weeks 0 days has been shown to be associated with an increased risk of learning disabilities and a potential increase in morbidity and mortality. There are clear medical indications for delivery prior to 39 weeks 0 days based on maternal and/or fetal conditions. A mature fetal lung test, in the absence of appropriate clinical criteria, is not an indication for delivery. Due to recently-published evidence related to induction of labor between 39 and 41 weeks gestation, the ACOG has withdrawn this 2 recommendation. Don’t perform routine annual cervical cytology screening (Pap tests) in women 30–65 years of age. 3 In average risk women, annual cervical cytology screening has been shown to offer no advantage over screening performed at 3-year intervals. However, a well-woman visit should occur annually for patients with their health care practitioner to discuss concerns and problems, and have appropriate screening with consideration of a pelvic examination. Don’t treat patients who have mild dysplasia of less than two years in duration. 4 Mild dysplasia (Cervical Intraepithelial Neoplasia [CIN 1]) is associated with the presence of the human papillomavirus (HPV), which does not require treatment in average risk women. Most women with CIN 1 on biopsy have a transient HPV infection that will usually clear in less than 12 months and, therefore, does not require treatment. -

Why Clinicians Overtest: Development of a Thematic Framework
Why clinicians overtest: development of a thematic framework Justin H Lam ( [email protected] ) The University of Sydney https://orcid.org/0000-0001-6076-3999 Kristen Pickles The University of Sydney Fiona Stanaway The University of Sydney Katy JL Bell The University of Sydney Research article Keywords: medical overuse, health service misuse, overtest, overtesting, clinician Posted Date: October 12th, 2020 DOI: https://doi.org/10.21203/rs.3.rs-43690/v2 License: This work is licensed under a Creative Commons Attribution 4.0 International License. Read Full License Version of Record: A version of this preprint was published on November 4th, 2020. See the published version at https://doi.org/10.1186/s12913-020-05844-9. Page 1/36 Abstract Background: Medical tests provide important information to guide clinical management. Overtesting, however, may cause harm to patients and the healthcare system, including through misdiagnosis, false positives, false negatives and overdiagnosis. Clinicians are ultimately responsible for test requests, and are therefore ideally positioned to prevent overtesting and its unintended consequences. Through this narrative literature review and workshop discussion with experts at the Preventing Overdiagnosis Conference (Sydney, 2019), we aimed to identify and establish a thematic framework of factors that inuence clinicians to request non-recommended and unnecessary tests. Methods: Articles exploring factors affecting clinician test ordering behaviour were identied through a systematic search of MedLine in April 2019, forward and backward citation searches and content experts. Two authors screened abstract titles and abstracts, and two authors screened full text for inclusion. Identied factors were categorised into a preliminary framework which was subsequently presented at the PODC for iterative development. -

Patient & Public Engagement in Choosing Wisely
PATIENT & PUBLIC ENGAGEMENT IN CHOOSING WISELY TOOLKIT | VERSION 1.0 Editors Anna Kurdina Karen Born Wendy Levinson Contributors Marco Bobbio Stephanie Callan Angela Coulter Moriah Ellen Amy Ma Robyn Lindner Paul Myres Ramai Santhirapala Todd Sikorski Choosing Wisely Canada receives support from The Commonwealth Fund, Bertelsmann Stiftung and Korea University to coordinate Choosing Wisely International. For more information, please contact [email protected] 2 CONTENTS BACKGROUND 4 Executive Summary 4 About Choosing Wisely International 7 Patient and Public Engagement 7 Framework 7 PARTNER 8 Case 1.1 - US: Partnership with Consumer Reports and 70 Other Organizations 9 Case 1.2 - Norway: Partnership with the Norwegian Patient Association 10 Case 1.3 - Canada: Patient Advisor Roles 11 Case 1.4 - Japan: Engagement Strategies with the Public nd Media 12 Case 1.5 - Italy: Partnerships with Altroconsumo and Partecipasalute 13 Case 1.6 - Australia: Consumer Stakeholder Roundtables 14 ENGAGE 15 Case 2.1 - New Zealand: Consumer Commentary Sessions 15 Case 2.2 - Canada: Canadian Rheumatology Association List 18 Case 2.3 - Israel: Recommendations from the Association of Family Physicians 19 Case 2.4 - UK: Specialty Societies Involving Patients 20 Case 2.5 - US: Tools to Engage Patients in QI Initiatives 21 Case 2.6 - Wales: “What matters to you/me” Day 22 Case 2.7 - Australia: Consumer Working Group 23 Case 2.8 - US: Community Conversations About Overuse 25 INFORM 26 Case 3.1 - Canada and New Zealand: Survey Data on Patient Attitudes -

Psychiatry.Pdf
Psychiatry Thirteen Things Physicians and Patients Should Question by Canadian Academy of Child and Adolescent Psychiatry Canadian Academy of Geriatric Psychiatry Canadian Psychiatric Association Last updated: June 2017 Don’t use atypical antipsychotics as a first-line intervention for insomnia in children and 1 youth. Recent research confirms a dramatic increase in the use of atypical antipsychotics with subsequent side-effects including obesity, which is already a major health issue. It is prudent to pursue nonpharmacological measures first, such as behavioural modifications and ensuring good sleep hygiene (such as eliminating daytime napping and shutting off electronics an hour before bedtime). If these interventions are not successful, then consider short-term use of melatonin. 2 Don’t use SSRIs as the first-line intervention for mild to moderately depressed teens. Evidence clearly indicates that antidepressant medication is less effective in children and adolescents up to the age of 17 years and first-line treatment for this group should include cognitive behavioural therapy or interpersonal psychotherapy. Attention should always be focused on children’s and teens’ environmental safety and adequate parental support to avoid missing cases of neglect or abuse. Following this, a first-line intervention should be psychoeducation on the importance of regular sleep, diet and exercise to ensure healthy, age-appropriate developmental support. 3 Don’t use atypical antipsychotics as a first-line intervention for Attention Deficit Hyperactivity Disorder (ADHD) with disruptive behaviour disorders. Treatment of ADHD should include adequate education of patients and their families, behavioural interventions, psychological treatments and educational accommodations first. If this approach is not sufficient, stimulant medication and a behavioural analysis to ensure appropriate support from the parent and classroom is indicated. -

Benzodiazepine Use Among Older Adults
Commentary For reprint orders, please contact: [email protected] Benzodiazepine use among older adults Aarti Gupta1 , Gargi Bhattacharya2 , Kripa Balaram3 , Deena Tampi4 & Rajesh R Tampi*,5,6 1Department of Psychiatry, Yale School of Medicine, New Haven, CT 06511, USA 2Department of Electrical and Computer Engineering, Purdue University, Indiana 47906, USA 3Department of Psychiatry, MetroHealth, Case Western Reserve University School of Medicine, Cleveland, OH 44109, USA 4Co-Founder and Managing Principal, Behavioral Health Advisory Group, Princeton, NJ 08542, USA 5Department of Psychiatry & Behavioral Sciences, Cleveland Clinic Akron General, Akron, OH 44307, USA 6Cleveland Clinic Lerner College of Medicine of Case Western Reserve University, Cleveland, OH 44195, USA *Author for correspondence: Tel.: +1 203 809 5223; [email protected] “the use of benzodiazepines is increasing among older adults and they form the largest group of users for these drugs. These agents are effective in treating some clinical symptoms, but their use is fraught with serious side effects and addiction potential among older adults.” First draft submitted: 26 October 2020; Accepted for publication: 29 October 2020; Published online: 11 November 2020 Keywords: adverse effects • benzodiazepines • cognitive decline • elderly • falls • older adults Benzodiazepines are one of the most commonly prescribed psychotropic drugs in the developed world. Recent data from the 2015 to 2016 National Survey on Drug Use and Health show 30.6 million adults reported benzodiazepine use in the past year. The highest use of benzodiazepines of all age groups was reported by respondents aged 50–65 years at 14.3%, followed closely by the ≥65 years age group at 12.9% [1]. -

3 1 2 5 4 Five Things Physicians and Patients
American Academy of Allergy, Asthma & Immunology Five Things Physicians and Patients Should Question Don’t perform unproven diagnostic tests, such as immunoglobulin G (IgG) testing or an indiscriminate battery of immunoglobulin E (IgE) 1 tests, in the evaluation of allergy. Appropriate diagnosis and treatment of allergies requires specific IgE testing (either skin or blood tests) based on the patient’s clinical history. The use of other tests or methods to diagnose allergies is unproven and can lead to inappropriate diagnosis and treatment. Appropriate diagnosis and treatment is both cost eective and essential for optimal patient care. Don’t order sinus computed tomography (CT) or indiscriminately prescribe antibiotics for uncomplicated acute rhinosinusitis. Viral infections cause the majority of acute rhinosinusitis and only 0.5 percent to 2 percent progress to bacterial infections. Most acute 2 rhinosinusitis resolves without treatment in two weeks. Uncomplicated acute rhinosinusitis is generally diagnosed clinically and does not require a sinus CT scan or other imaging. Antibiotics are not recommended for patients with uncomplicated acute rhinosinusitis who have mild illness and assurance of follow-up. If a decision is made to treat, amoxicillin should be first-line antibiotic treatment for most acute rhinosinsutis. Don’t routinely do diagnostic testing in patients with chronic urticaria. In the overwhelming majority of patients with chronic urticaria, a definite etiology is not identified. Limited laboratory testing may be warranted to 3 exclude underlying causes. Targeted laboratory testing based on clinical suspicion is appropriate. Routine extensive testing is neither cost eective nor associated with improved clinical outcomes. Skin or serum-specific IgE testing for inhalants or foods is not indicated, unless there is a clear history implicating an allergen as a provoking or perpetuating factor for urticaria. -

Choosing Wisely? Quantifying the Extent of Three Low Value Psychotropic Prescribing Practices in Australia Jonathan Brett1* , Helga Zoega1,2, Nicholas A
View metadata, citation and similar papers at core.ac.uk brought to you by CORE provided by Opin visindi Brett et al. BMC Health Services Research (2018) 18:1009 https://doi.org/10.1186/s12913-018-3811-5 RESEARCHARTICLE Open Access Choosing wisely? Quantifying the extent of three low value psychotropic prescribing practices in Australia Jonathan Brett1* , Helga Zoega1,2, Nicholas A. Buckley3, Benjamin J. Daniels1, Adam G. Elshaug4 and Sallie-Anne Pearson1,4 Abstract Background: The global Choosing Wisely campaign has identified the following psychotropic prescribing as low-value (harmful or wasteful): (1) benzodiazepine use in the elderly,(2)antipsychotic use in dementia and (3) prescribing two or more antipsychotics concurrently. We aimed to quantify the extent of these prescribing practices in the Australian population. Methods: We applied indicators to dispensing claims of a 10% random sample of Australian Pharmaceutical Benefits Scheme beneficiaries to quantify annual rates of each low-value practice from 2013 to 2016. We also assessed patient factors and direct medicine costs (extrapolated to the entire Australian population) associated with each practice in 2016. Results: We observed little change in the rates of the three practices between 2013 and 2016. In 2016, 15.3% of people aged ≥65 years were prescribed a benzodiazepine, 0.5% were prescribed antipsychotics in the context of dementia and 0.2% of people aged ≥18 years received two or more antipsychotics concurrently. The likelihood of elderly people receiving benzodiazepines or antipsychotics in the context of dementia increased with age and the likelihood of receiving all three practices increased with comorbidity burden. -
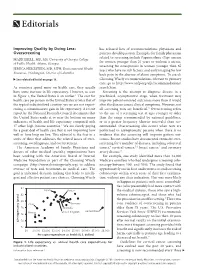
Improving Quality by Doing Less: Has Released Lists of Recommendations Physicians and Overscreening Patients Should Question
Editorials Improving Quality by Doing Less: has released lists of recommendations physicians and Overscreening patients should question. Examples for family physicians related to screening include Papanicolaou (Pap) smears MARK EBELL, MD, MS, University of Georgia College for women younger than 21 years or without a uterus, of Public Health, Athens, Georgia screening for osteoporosis in women younger than 65 JESSICA HERZSTEIN, MD, MPH, Environmental Health years who have no risk factors, and early imaging for low Resources, Washington, District of Columbia back pain in the absence of alarm symptoms. To search ▲ See related editorial on page 18. Choosing Wisely recommendations relevant to primary care, go to http://www.aafp.org/afp/recommendations/ As countries spend more on health care, they usually search.htm. have some increase in life expectancy. However, as seen Screening is the attempt to diagnose disease in a in Figure 1, the United States is an outlier.1 The cost for preclinical, asymptomatic stage, when treatment may health care per person in the United States is twice that of improve patient-oriented outcomes more than it would the typical industrialized country, yet we are not experi- after the disease causes clinical symptoms. However, not encing a commensurate gain in life expectancy. A recent all screening tests are beneficial.3 Overscreening refers report by the National Research Council documents that to the use of a screening test at ages younger or older the United States ranks at or near the bottom on many than the range recommended by national guidelines, indicators of health and life expectancy compared with or at a greater frequency (shorter intervals) than rec- 17 other high-income countries.2 We are clearly paying ommended. -

Tackling the Epidemic of Low-Value Spending and Medical Overuse: Opportunities for Purchasers and Carriers
Tackling the Epidemic of Low-Value Spending and Medical Overuse: Opportunities for Purchasers and Carriers Draft Document Contents 1. INTRODUCTION ................................................................................................................................... 2 1-A. TYPES OF WASTE ......................................................................................................................... 3 CLINICAL WASTE – LOW-VALUE CARE/OVERUSE .............................................................................. 3 CLINICAL WASTE – OPERATIONAL WASTE ........................................................................................ 4 ADMINISTRATIVE WASTE ................................................................................................................. 4 Sidebar: Choosing Wisely ................................................................................................................. 4 1-B. SALIENCE AND SIGNIFICANCE OF LOW-VALUE CARE ACROSS STAKEHOLDERS ............................ 6 PATIENTS ......................................................................................................................................... 6 PAYERS, PURCHASERS, AND CARRIERS ........................................................................................... 15 PROVIDERS .................................................................................................................................... 18 Sidebar: Tort Reform and Defensive Medicine .............................................................................. -

From Polypharmacy to Deprescribing: the Wise Choice
From Polypharmacy to Deprescribing: A Wise Choice Donna Bartlett PharmD, BCGP [email protected] June 2019 Objectives • Define polypharmacy, how it can negatively affect the older population, including potential harms • Detect health issues that may be caused by multiple prescribed and over the counter medications; address these issues appropriately • Enumerate deprescribing opportunities including alternative therapies to medications to provide optimal prescribing Choosing Wisely • Don’t use antipsychotics as the first choice to treat behavioral and psychological symptoms of dementia. • Avoid using medications other than metformin to achieve hemoglobin A1c <7.5% in most older adults; moderate control is generally better. • Don’t use benzodiazepines or other sedative-hypnotics in older adults as first choice for insomnia, agitation or delirium. • Don’t prescribe cholinesterase inhibitors for dementia without periodic assessment for perceived cognitive benefits and adverse gastrointestinal effects. • Don’t prescribe a medication without conducting a drug regimen review. Polypharmacy Add in a hospitalization Multiple medications Multiple Multiple pharmacies prescribers Over the Supplements counter and herbals medications Polypharmacy • Definition: • Multiple medications (4, 5, 6, 7, 8…) • Administration of unnecessary medications • Hutchinson, Sleeper 2010. Polypharmacy Risk Factors: • Demographics Increased age, White, Educated • Health status Poorer health, depression, HTN, anemia, asthma, angina, diabetes, gout, osteoarthritis • -

Hospital Pharmacy
Hospital Pharmacy Six Things Clinicians and Patients Should Question by Canadian Society of Hospital Pharmacists Last updated: January 2019 Don’t continue medications that are no longer indicated or where the risks outweigh the 1 benefits. Polypharmacy, often defined as taking five or more medications at the same time, has been associated with a variety of adverse health outcomes. Therapy with a medication is initiated when the patient and care team conclude that the benefits of taking the medication outweigh the risks of not starting therapy. However, over time, patients and their conditions or goals of care change, new evidence is discovered, and other factors can tip the balance, such that the benefits no longer outweigh the risks or burdens of continued treatment. Few, if any, medications should be continued on a lifelong basis. Patients and caregivers should be made aware of the planned duration of therapy and the outcomes desired, and should feel empowered to follow up with providers to ensure that the benefits of therapy continue to outweigh the risks. The performance of medication reconciliation and transitions of care—such as admission to or discharge from a hospital—may serve as critical activities for deciding whether to continue therapy or create a plan to safely stop a medication. 2 Don’t use a medication for long-term risk reduction if life expectancy is shorter than the time to benefit of the medication. The “time to benefit” is the period between initiation of an intervention (in this case, a medication) and the point when the patient begins to experience a benefit.