The Species Declared Extinct in 2020 by John R
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Conservation of Handfish and Their Habitats – Annual Report Tim Lynch, Tyson Bessell, Alexander Hormann, Carlie Devine and Neville Barrett
Conservation of handfish and their habitats – annual report Tim Lynch, Tyson Bessell, Alexander Hormann, Carlie Devine and Neville Barrett Project A10 – Conservation of spotted handfish 28 February 2019 Milestone 4– Research Plan 4 (2018) www.nespmarine.edu.au Enquiries should be addressed to: Dr Tim P. Lynch Senior Research Scientist CSIRO Castray Esplanade [email protected] Project Leader’s Distribution List Derwent Estuary Program Ursula Taylor Zoo and Aquarium Association (ZAA) Craig Thorburn Natural Resource Management (NRM) Nepelle Crane South MAST Ian Ross Royal Yacht Club of Tasmania Nick Hutton Derwent Sailing Squadron Shaun Tiedemann The Handfish Recovery Team (HRT) See list below Marine and Freshwater Species Conservation Section Wildlife, Heritage and Marine Division Department of the Environment and Energy (DoEE) Threatened Species Policy and Andrew Crane Conservation Advice Branch Department of Primary Industries, Parks, Water and Environment (DPIPWE) Office of the Threatened Species Commissioner (DoEE) The project will also report its findings on a semi-annual basis to the National Handfish Recovery Team (NHRT) – see below. This is a governance body that is constituted between the Tasmanian State and the Commonwealth government with other interested parties: Department of the Environment and Energy (Commonwealth) Department of Primary Industries, Parks, Water and Andrew Crane Environment (Tas) CSIRO scientist, running current surveys and substrate trials Tim Lynch (Chair) University of Tasmania, handfish research Neville -
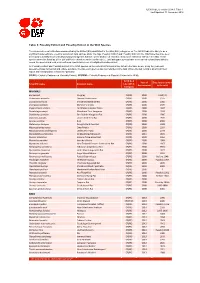
Table 9: Possibly Extinct and Possibly Extinct in the Wild Species
IUCN Red List version 2014.3: Table 9 Last Updated: 13 November 2014 Table 9: Possibly Extinct and Possibly Extinct in the Wild Species The number of recent extinctions documented by the Extinct (EX) and Extinct in the Wild (EW) categories on The IUCN Red List is likely to be a significant underestimate, even for well-known taxa such as birds. The tags 'Possibly Extinct' and 'Possibly Extinct in the Wild' have therefore been developed to identify those Critically Endangered species that are, on the balance of evidence, likely to be extinct (or extinct in the wild). These species cannot be listed as EX or EW until their extinction can be confirmed (i.e., until adequate surveys have been carried out and have failed to record the species and local or unconfirmed reports have been investigated and discounted). All 'Possibly Extinct' and 'Possibly Extinct in the Wild' species on the current IUCN Red List are listed in the table below, along the year each assessment was carried out and, where available, the date each species was last recorded in the wild. Where the last record is an unconfirmed report, last recorded date is noted as "possibly". CR(PE) - Critically Endangered (Possibly Extinct), CR(PEW) - Critically Endangered (Possibly Extinct in the Wild), IUCN Red Year of Date last recorded Scientific name Common name List (2014) Assessment in the wild Category MAMMALS Bos sauveli Kouprey CR(PE) 2008 1969/70 Crateromys australis Dinagat Crateromys CR(PE) 2008 1975 Crocidura trichura Christmas Island Shrew CR(PE) 2008 1985 Crocidura wimmeri -
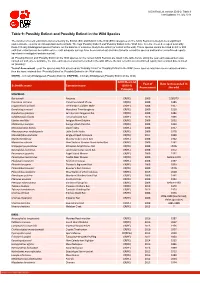
Table 9: Possibly Extinct and Possibly Extinct in the Wild Species
IUCN Red List version 2019-2: Table 9 Last Updated: 18 July 2019 Table 9: Possibly Extinct and Possibly Extinct in the Wild Species The number of recent extinctions documented by the Extinct (EX) and Extinct in the Wild (EW) categories on The IUCN Red List is likely to be a significant underestimate, even for well-known taxa such as birds. The tags 'Possibly Extinct' and 'Possibly Extinct in the Wild' have therefore been developed to identify those Critically Endangered species that are, on the balance of evidence, likely to be extinct (or extinct in the wild). These species cannot be listed as EX or EW until their extinction can be confirmed (i.e., until adequate surveys have been carried out and have failed to record the species and local or unconfirmed reports have been investigated and discounted). All 'Possibly Extinct' and 'Possibly Extinct in the Wild' species on the current IUCN Red List are listed in the table below, along the year each assessment was carried out and, where available, the date each species was last recorded in the wild. Where the last record is an unconfirmed report, last recorded date is noted as "possibly". Year of Assessment - year the species was first assessed as 'Possibly Extinct' or 'Possibly Extinct in the Wild'; some species may have been reassessed since then but have retained their 'Possibly Extinct' or 'Possibly Extinct in the Wild' status. CR(PE) - Critically Endangered (Possibly Extinct), CR(PEW) - Critically Endangered (Possibly Extinct in the Wild), IUCN Red List Year of Date last recorded in -

Conserving Critically Endangered Spotted Handfish
Conserving Critically Endangered spotted handfish Unique and quirky, spotted handfish (Brachionichthys hirsutus) are recognisable by their modified fins that resemble human hands. Once common in southern Tasmania’s Derwent estuary, spotted handfish experienced a severe decline in the 1980s. In 1996 they became the first marine fish to be listed as Critically Endangered by the IUCN Red List of Threatened Species. They are also listed as Critically Endangered under the Commonwealth Environment Protection and Biodiversity Conservation Act 1999, and Endangered under Tasmania's Threatened Species Protection Act 1995. Spotted handfish were once common in Tasmania’s Derwent estuary, but their populations experienced CSIRO, University of Tasmania (UTAS), the a serious decline in the 1980s. Tasmanian and Australian governments and the Derwent Estuary Program (DEP) have between 5–15m. Within bays they occupy been working together to conserve spotted habitats with more complex features such as handfish since the mid-1990s. depressions in the seabed made by stingrays, or fields of sea-squirts. Distribution Lacking swim bladders, spotted handfish use Handfish belong to a group of coastal their modified fins to ‘walk’ across the seabed anglerfish with a narrow distribution in south- rather than swim. Movement studies suggest eastern Australia. There are 14 species with they only travel small distances: 10m–460m seven endemic to Tasmania and Bass Strait. over many months, or an average of 4m a day. Spotted handfish were once prevalent along Spotted handfish are ambush predators and, Tasmania’s eastern coast, and were so like their close cousins the deep-sea angler common that during the 1960s and ‘70s that fishes, they have a lure located just above the they were routinely collected for practical mouth, perhaps to entice their prey of demonstrations at Hobart’s university. -

Handfish Survey in the Vicinity of a Proposed Intake Pipeline
HANDFISH SURVEY IN THE VICINITY OF A PROPOSED INTAKE PIPELINE AND EXISTING INTAKE INSPECTION AT CRAYFISH POINT, TAROONA, DERWENT ESTUARY Report to IMAS March 2018 www.marinesolutions.net.au © Marine Solutions 2018. This document should only be used for the specific project and purposes for which it was commissioned. 1 Version Author Date Reviewed by reviewed 1 of 1 Annie Ford and 20/03/2018 Laura Smith Joanna Smart Note: Location maps throughout this report are representative only; for precise GPS coordinates, see the appendices. All satellite imagery used throughout is sourced from The Land Information System Tasmania (LIST). 1 Cover photo, IMAS Taroona, November 2016 (photo by Marine Solutions). Handfish survey at site of proposed intake pipeline, Taroona 2 TABLE OF CONTENTS Table of Contents .......................................................................................................................................... 3 Table of Figures ............................................................................................................................................. 5 1 Executive Summary ............................................................................................................................... 6 2 Introduction .......................................................................................................................................... 8 2.1 Background .................................................................................................................................. -

New Opportunities for Conservation of Handfishes
Biological Conservation 208 (2017) 174–182 Contents lists available at ScienceDirect Biological Conservation journal homepage: www.elsevier.com/locate/bioc New opportunities for conservation of handfishes (Family Brachionichthyidae) and other inconspicuous and threatened marine species through citizen science Graham J. Edgar a,⁎,RickD.Stuart-Smitha, Antonia Cooper a, Michael Jacques b, Joe Valentine c a Institute for Marine and Antarctic Studies, University of Tasmania, Hobart, Tasmania 7001, Australia b Marine Life Network, 12 Blessington Street, South Arm, Tasmania 7022, Australia c Aquenal Ptd Ltd., Summerleas Rd, Kingston, Tasmania, Australia article info abstract Article history: Volunteer divers participating in the Reef Life Survey (RLS) program actively assist species conservation efforts by Received 15 October 2015 generating data for threat assessments and population trend monitoring, through in-water restoration efforts, Received in revised form 16 July 2016 and through outreach of marine conservation messages. Up to 2014, standardised underwater visual survey Accepted 22 July 2016 data provided by RLS divers described densities of 495 cryptic fish species at over 1200 sites distributed around Available online 9 August 2016 Australia. Each species was recorded on 34 separate transect blocks on average, allowing the first assessments of population trends for many species. These data highlight the threatened and data deficient status of endemic Keywords: fi fi fi Population monitoring Australian hand sh species. At least ve shallow-water hand sh species are potentially threatened, including Reef Life Survey the smooth handfish Sympterichthys unipennis, which has not been sighted for over 200 years, but is yet to be in- State-of-the-environment reporting cluded on any threatened species list. -

Nursery Rearing of Thai Sarpunti, Barbonymus Gonionotus Larvae Using Three Different Supplementary Feeds
J. Bangladesh Agril. Univ. 7(1): 139–144, 2009 ISSN 1810-3030 Nursery rearing of Thai sarpunti, Barbonymus gonionotus larvae using three different supplementary feeds A. K. S. Ahammad, M. M. R. Khan, M. A. Hossain1 and I. Parvez2 Department of Fisheries Biology and Genetics, Bangladesh Agricultural University, Mymensingh-2202, Bangladesh 1Department of Biotechnology, Bangladesh Agricultural University, Mymensingh-2202, Bangladesh 2Department of Fisheries Biology and Genetics, Hajee Mohammad Danesh Science and Technology University, Dinajpur, Bangladesh Abstract Nursery rearing of silver barb, Puntius gonionotus (Bleeker, 1850) larvae was carried out with three different feeding treatments T1, T2 and T3 having three replications each in nine rectangular glass aquaria (45x25x24 cm) for a period of 28 days in laboratory condition. Live planktonic feed (5000 cells/L), plankton and rice bran having 14.14% protein, and plankton and Saudi-Bangla nursery feed having 30.20% protein were tested as T1, T2 and T3, respectively. Three days old larvae of B. gonionotus (average length 5.0±0.15 mm and weight 7.0±0.05 mg) were stocked at a stocking density of 4.1 larvae/L of water in each aquarium. The highest length at harvest (28.06±0.38 mm and weight 135.00±3.05 mg) and also highest SGR (18.79±0.80) were found in T3 followed by T2 and T1. The survival rate in all the treatments was high (92-90%) and treatment to treatment variation was not significant (P<0.05). The result implies that the application of supplemental feeds over control in nursery rearing of B. -

Asterias Amurensis Global Invasive
FULL ACCOUNT FOR: Asterias amurensis Asterias amurensis System: Marine Kingdom Phylum Class Order Family Animalia Echinodermata Asteroidea Forcipulatida Asteriidae Common name North Pacific seastar (English), Nordpazifischer Seestern (German), Japanese seastar (English), northern Pacific seastar (English), purple-orange seastar (English), flatbottom seastar (English), Japanese starfish (English) Synonym Parasterias albertensis , Verrill, 1914 Asterias rubens , Murdoch, 1885 Asterias pectinata , Brandt, 1835 Asterias nortonensis , Clark, 1920 Asterias anomala , Clark, 1913 Asterias amurensis , f. robusta Djakonov, 1950 Asterias amurensis , f. latissima Djakonov, 1950 Allasterias rathbuni nortonens , Verrill, 1909 Allasterias rathbuni , var. anom Verrill, 1909 Allasterias rathbuni , var. nort Verrill, 1914 Asterias amurensis , f. acervispinis Djakonov, 1950 Asterias amurensis , f. flabellifera Djakonov, 1950 Asterias amurensis , f. gracilispinis Djakonov, 1950 Similar species Pisaster brevispinus, Pisaster giganteus, Pisaster ochraceus Summary Originally found in far north Pacific waters and areas surrounding Japan, Russia, North China, and Korea, the northern Pacific seastar (Asterias amurensis) has successfully invaded the southern coasts of Australia and has the potential to move as far north as Sydney. The seastar will eat a wide range of prey and has the potential for ecological and economic harm in its introduced range. Because the seastar is well established and abundantly widespread, eradication is almost impossible. However, prevention and control measures are being implemented to stop the species from establishing in new waters. view this species on IUCN Red List Global Invasive Species Database (GISD) 2021. Species profile Asterias amurensis. Pag. 1 Available from: http://www.iucngisd.org/gisd/species.php?sc=82 [Accessed 06 October 2021] FULL ACCOUNT FOR: Asterias amurensis Species Description Asterias amurensis (northern Pacific seastar) can grow upto 50cm in diameter. -

Carnatic Carp (Barbodes Carnaticus) ERSS
Carnatic Carp (Barbodes carnaticus) Ecological Risk Screening Summary U.S. Fish & Wildlife Service, March 2015 Revised, September 2017, October 2017 Web Version, 8/28/2018 Photo: Dr. N. Basavaraja. Licensed under Creative Commons BY-NC 3.0. Available: http://www.fishbase.org/photos/UploadedBy.php?autoctr=12615&win=uploaded. (March 30, 2015). 1 Native Range and Status in the United States Native Range From Ali and Raghavan (2013): “Barbodes carnaticus is endemic to the Western Ghats (Dahanukar et al. 2004). Known from rivers in the states of Kerala, Tamil Nadu and Karnataka including Cauvery, Krishna (Jayaram 1999), Moyar (Rajan 1963, Arunachalam et al. 2000), Kabini, Bhavani, Bharathapuzha, 1 Chalakudy, Periyar, Pambar, Muvattupuzha, Manimala, Pamba, Achenkovil, Karamana, Neyyar (Shaji and Easa 2003, Chhapgar and Mankadan 2000, Kurup et al. 2004), Chaliyar (R. Raghavan and A. Ali pers. obs.). Ooty Lake (Jayaram 1999). Besides it has also been reported from the water bodies inside the Mudumalai Wildlife Sanctuary (Manimekalan 1998), from the drainages in the Dharmapuri district of Tamil Nadu (Rema Devi and Raghunathan 1999) and from Kolli Hills of Eastern Ghats (Arunachalam and Johnson 1998). The record from Tambraparini (Johnsingh and Vickram 1987) is erroneous (Johnson and Arunachalam pers. comm.). The report from southern Kerala is also doubtful (M. Arunachalam pers. comm.).” Status in the United States No records of Barbodes carnaticus in the wild or in trade in the United States were found. Means of Introductions in the United States No records of Barbodes carnaticus in the United States were found. Remarks No additional remarks. 2 Biology and Ecology Taxonomic Hierarchy and Taxonomic Standing According to Eschmeyer et al. -

Baby Red Handfish
Wednesday 4 December 2019 **News editors note: media can see the red handfish and interview researchers today at 11 am, IMAS Taroona, 15-21 Nubeena Crescent** Tiny red handfish hatchlings a lifeline for world’s rarest fish Fifty newborn red handfish are giving Institute for Marine and Antarctic Studies (IMAS) scientists an opportunity to help save the last known populations of the world’s rarest fish. The tiny red handfish hatched in an IMAS aquarium this month from two egg masses collected at one of the last remaining sites in southern Tasmania where fewer than 100 adults survive. IMAS researcher Dr Jemina Stuart-Smith said keeping the juveniles in a safe environment during their vulnerable early stages would protect them from predators and environmental risks. “These juvenile red handfish will play a vital role in ensuring the species continues to survive in the wild,” Dr Stuart-Smith said. “We plan to release them back into their remaining habitat when they are around one-year-old, to help rebuild the population at one of the two known sites that have been compromised by range of impacts, including habitat loss. “Raising them in a controlled environment is a conservation strategy known as headstarting, designed to improve their chances of surviving to maturity and eventually reproducing. “Little is known about red handfish biology, reproduction and early growth, and these juveniles will also allow critical research that can help us to ensure this is not the last generation of their species,” Dr Stuart-Smith said. IMAS PhD student Tyson Bessell said handfish lay eggs on upright stalks of vegetation on the seafloor and the mother stays with them until they hatch. -

Guide to Theecological Systemsof Puerto Rico
United States Department of Agriculture Guide to the Forest Service Ecological Systems International Institute of Tropical Forestry of Puerto Rico General Technical Report IITF-GTR-35 June 2009 Gary L. Miller and Ariel E. Lugo The Forest Service of the U.S. Department of Agriculture is dedicated to the principle of multiple use management of the Nation’s forest resources for sustained yields of wood, water, forage, wildlife, and recreation. Through forestry research, cooperation with the States and private forest owners, and management of the National Forests and national grasslands, it strives—as directed by Congress—to provide increasingly greater service to a growing Nation. The U.S. Department of Agriculture (USDA) prohibits discrimination in all its programs and activities on the basis of race, color, national origin, age, disability, and where applicable sex, marital status, familial status, parental status, religion, sexual orientation genetic information, political beliefs, reprisal, or because all or part of an individual’s income is derived from any public assistance program. (Not all prohibited bases apply to all programs.) Persons with disabilities who require alternative means for communication of program information (Braille, large print, audiotape, etc.) should contact USDA’s TARGET Center at (202) 720-2600 (voice and TDD).To file a complaint of discrimination, write USDA, Director, Office of Civil Rights, 1400 Independence Avenue, S.W. Washington, DC 20250-9410 or call (800) 795-3272 (voice) or (202) 720-6382 (TDD). USDA is an equal opportunity provider and employer. Authors Gary L. Miller is a professor, University of North Carolina, Environmental Studies, One University Heights, Asheville, NC 28804-3299. -

Barbodes Manalak Ecological Risk Screening Summary
Barbodes manalak (a fish, no common name) Ecological Risk Screening Summary U.S. Fish and Wildlife Service, November 2013 Revised, July 2018 Web Version, 8/20/2018 1 Native Range and Status in the United States Native Range From Froese and Pauly (2018): “Asia: endemic to Lake Lanao, Mindanao, Philippines.” Status in the United States This species has not been reported as introduced or established in the United States. There is no indication that this species is in trade in the United States. Means of Introductions in the United States This species has not been reported as introduced or established in the United States. 1 Remarks From World Conservation Monitoring Centre (1996): “Harrison and Stiassny (1999) consider this species to be possibly extinct. The matter has been referred to the relevant Specialist Group for a decision.” A previous version of this ERSS was drafted under the name Puntius manalak, which was the previously accepted name of this species. The currently accepted name is Barbodes manalak. Both names were used when researching in preparation of this report. 2 Biology and Ecology Taxonomic Hierarchy and Taxonomic Standing From ITIS (2018): “Kingdom Animalia Subkingdom Bilateria Infrakingdom Deuterostomia Phylum Chordata Subphylum Vertebrata Infraphylum Gnathostomata Superclass Actinopterygii Class Teleostei Superorder Ostariophysi Order Cypriniformes Superfamily Cyprinoidea Family Cyprinidae Genus Puntius Species Puntius manalak (Herre, 1924)” From Eschmeyer et al. (2018): “Current status: Valid as Barbodes manalak Herre 1924. Cyprinidae: Smiliogastrinae.” Size, Weight, and Age Range From Froese and Pauly (2018): “Max length : 31.5 cm TL male/unsexed; [Herre 1924]” Environment From Froese and Pauly (2018): “Freshwater; benthopelagic” 2 Climate/Range From Froese and Pauly (2018): “Tropical” Distribution Outside the United States Native From Froese and Pauly (2018): “Asia: endemic to Lake Lanao, Mindanao, Philippines.” Introduced This species has not been reported as introduced or established outside of its native range.