Western Birds-43(4)-Webcomp.Pdf
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Costa Rica 2020
Sunrise Birding LLC COSTA RICA TRIP REPORT January 30 – February 5, 2020 Photos: Talamanca Hummingbird, Sunbittern, Resplendent Quetzal, Congenial Group! Sunrise Birding LLC COSTA RICA TRIP REPORT January 30 – February 5, 2020 Leaders: Frank Mantlik & Vernon Campos Report and photos by Frank Mantlik Highlights and top sightings of the trip as voted by participants Resplendent Quetzals, multi 20 species of hummingbirds Spectacled Owl 2 CR & 32 Regional Endemics Bare-shanked Screech Owl 4 species Owls seen in 70 Black-and-white Owl minutes Suzy the “owling” dog Russet-naped Wood-Rail Keel-billed Toucan Great Potoo Tayra!!! Long-tailed Silky-Flycatcher Black-faced Solitaire (& song) Rufous-browed Peppershrike Amazing flora, fauna, & trails American Pygmy Kingfisher Sunbittern Orange-billed Sparrow Wayne’s insect show-and-tell Volcano Hummingbird Spangle-cheeked Tanager Purple-crowned Fairy, bathing Rancho Naturalista Turquoise-browed Motmot Golden-hooded Tanager White-nosed Coati Vernon as guide and driver January 29 - Arrival San Jose All participants arrived a day early, staying at Hotel Bougainvillea. Those who arrived in daylight had time to explore the phenomenal gardens, despite a rain storm. Day 1 - January 30 Optional day-trip to Carara National Park Guides Vernon and Frank offered an optional day trip to Carara National Park before the tour officially began and all tour participants took advantage of this special opportunity. As such, we are including the sightings from this day trip in the overall tour report. We departed the Hotel at 05:40 for the drive to the National Park. En route we stopped along the road to view a beautiful Turquoise-browed Motmot. -

Management Plan for the Yellow Rail in Canada
Species at Risk Actl Management Plan Series Management Plan for the Yellow Rail (Coturnicops noveboracensis) in Canada Yellow Rail 2013 Recommended citation: Environment Canada. 2013. Management Plan for the Yellow Rail (Coturnicops noveboracensis) in Canada. Species at Risk Act Management Plan Series. Environment Canada, Ottawa. iii + 24 pp. For copies of the management plan, or for additional information on species at risk, including COSEWIC Status Reports, residence descriptions, action plans, and other related recovery documents, please visit the Species at Risk (SAR) Public Registry (www.sararegistry.gc.ca). Cover photo: ©Jacques Brisson Également disponible en français sous le titre « Plan de gestion du Râle jaune (Coturnicops noveboracensis) au Canada » © Her Majesty the Queen in Right of Canada, represented by the Minister of the Environment, 2013. All rights reserved. ISBN 978-1-100-21199-2 Catalogue no. En3-5/38-2013E-PDF Content (excluding the illustrations) may be used without permission, with appropriate credit to the source. Management Plan for the Yellow Rail 2013 PREFACE The federal, provincial, and territorial government signatories under the Accord for the Protection of Species at Risk (1996) agreed to establish complementary legislation and programs that provide for effective protection of species at risk throughout Canada. Under the Species at Risk Act (S.C. 2002, c.29) (SARA), the federal competent ministers are responsible for the preparation of management plans for listed Special Concern species and are required to report on progress within five years. The Minister of the Environment and the Minister responsible for the Parks Canada Agency are the competent ministers for the management of the Yellow Rail and have prepared this plan, as per section 65 of SARA. -

Rare Birds of California Now Available! Price $54.00 for WFO Members, $59.99 for Nonmembers
Volume 40, Number 3, 2009 The 33rd Report of the California Bird Records Committee: 2007 Records Daniel S. Singer and Scott B. Terrill .........................158 Distribution, Abundance, and Survival of Nesting American Dippers Near Juneau, Alaska Mary F. Willson, Grey W. Pendleton, and Katherine M. Hocker ........................................................191 Changes in the Winter Distribution of the Rough-legged Hawk in North America Edward R. Pandolfino and Kimberly Suedkamp Wells .....................................................210 Nesting Success of California Least Terns at the Guerrero Negro Saltworks, Baja California Sur, Mexico, 2005 Antonio Gutiérrez-Aguilar, Roberto Carmona, and Andrea Cuellar ..................................... 225 NOTES Sandwich Terns on Isla Rasa, Gulf of California, Mexico Enriqueta Velarde and Marisol Tordesillas ...............................230 Curve-billed Thrasher Reproductive Success after a Wet Winter in the Sonoran Desert of Arizona Carroll D. Littlefield ............234 First North American Records of the Rufous-tailed Robin (Luscinia sibilans) Lucas H. DeCicco, Steven C. Heinl, and David W. Sonneborn ........................................................237 Book Reviews Rich Hoyer and Alan Contreras ...........................242 Featured Photo: Juvenal Plumage of the Aztec Thrush Kurt A. Radamaker .................................................................247 Front cover photo by © Bob Lewis of Berkeley, California: Dusky Warbler (Phylloscopus fuscatus), Richmond, Contra Costa County, California, 9 October 2008, discovered by Emilie Strauss. Known in North America including Alaska from over 30 records, the Dusky is the Old World Warbler most frequent in western North America south of Alaska, with 13 records from California and 2 from Baja California. Back cover “Featured Photos” by © Kurt A. Radamaker of Fountain Hills, Arizona: Aztec Thrush (Ridgwayia pinicola), re- cently fledged juvenile, Mesa del Campanero, about 20 km west of Yecora, Sonora, Mexico, 1 September 2007. -

Hungary & Transylvania
Although we had many exciting birds, the ‘Bird of the trip’ was Wallcreeper in 2015. (János Oláh) HUNGARY & TRANSYLVANIA 14 – 23 MAY 2015 LEADER: JÁNOS OLÁH Central and Eastern Europe has a great variety of bird species including lots of special ones but at the same time also offers a fantastic variety of different habitats and scenery as well as the long and exciting history of the area. Birdquest has operated tours to Hungary since 1991, being one of the few pioneers to enter the eastern block. The tour itinerary has been changed a few times but nowadays the combination of Hungary and Transylvania seems to be a settled and well established one and offers an amazing list of European birds. This tour is a very good introduction to birders visiting Europe for the first time but also offers some difficult-to-see birds for those who birded the continent before. We had several tour highlights on this recent tour but certainly the displaying Great Bustards, a majestic pair of Eastern Imperial Eagle, the mighty Saker, the handsome Red-footed Falcon, a hunting Peregrine, the shy Capercaillie, the elusive Little Crake and Corncrake, the enigmatic Ural Owl, the declining White-backed Woodpecker, the skulking River and Barred Warblers, a rare Sombre Tit, which was a write-in, the fluty Red-breasted and Collared Flycatchers and the stunning Wallcreeper will be long remembered. We recorded a total of 214 species on this short tour, which is a respectable tally for Europe. Amongst these we had 18 species of raptors, 6 species of owls, 9 species of woodpeckers and 15 species of warblers seen! Our mammal highlight was undoubtedly the superb views of Carpathian Brown Bears of which we saw ten on a single afternoon! 1 BirdQuest Tour Report: Hungary & Transylvania 2015 www.birdquest-tours.com We also had a nice overview of the different habitats of a Carpathian transect from the Great Hungarian Plain through the deciduous woodlands of the Carpathian foothills to the higher conifer-covered mountains. -

First Documented Observation of Sedge Wren in Arizona Accepted
Arizona Birds - Journal of Arizona Field Ornithologists Volume 2011 FIRST DOCUMENTED OBSERVATION OF SEDGE WREN (Cistothorus platensis) IN ARIZONA Alan Schmierer, PO Box 626, Patagonia, AZ 85624 ([email protected]) Photos by the author. On 27 November 2010 the author discovered and photographed a Sedge Wren (Cistothorus platensis) on the shores of Peña Blanca Lake, Santa Cruz County, Arizona. This sighting was the first documented record of this species for Arizona. INITIAL DISCOVERY AND CONTINUED SIGHTINGS The initial discovery of this bird was a very brief encounter. At about mid-morning I was birding the south shore of the cove (informally called Thumb Rock Cove; see Figure 2) that is just north of the Upper Thumb Rock Picnic Area parking lot. Several double-chip notes, some- what reminiscent of those of the Pacific and Winter Wrens (Troglodytes pacificus and heimalis) alerted me to a potentially interesting bird be- ing present, followed by a 15 second look at the bird with binoculars (at about a two meter dis- tance), two quick photos (a lesson for photogra- phers to keep their cameras handy for such “bird emergencies”) and then the bird was gone. The wren was seen while it was about 1.5 m up in bare branches close to the trunk of a small deciduous tree at the water’s edge, and it then flew across the cove to a grassy edge of the op- posite shore. That brief view was enough for me to identify it as a Sedge Wren. Even as a new- comer to Arizona I knew that it was likely a very Figure 1: SEDGE WREN (27 November 2010) at initial sighting. -

A Comprehensive Multilocus Assessment of Sparrow (Aves: Passerellidae) Relationships ⇑ John Klicka A, , F
Molecular Phylogenetics and Evolution 77 (2014) 177–182 Contents lists available at ScienceDirect Molecular Phylogenetics and Evolution journal homepage: www.elsevier.com/locate/ympev Short Communication A comprehensive multilocus assessment of sparrow (Aves: Passerellidae) relationships ⇑ John Klicka a, , F. Keith Barker b,c, Kevin J. Burns d, Scott M. Lanyon b, Irby J. Lovette e, Jaime A. Chaves f,g, Robert W. Bryson Jr. a a Department of Biology and Burke Museum of Natural History and Culture, University of Washington, Box 353010, Seattle, WA 98195-3010, USA b Department of Ecology, Evolution, and Behavior, University of Minnesota, 100 Ecology Building, 1987 Upper Buford Circle, St. Paul, MN 55108, USA c Bell Museum of Natural History, University of Minnesota, 100 Ecology Building, 1987 Upper Buford Circle, St. Paul, MN 55108, USA d Department of Biology, San Diego State University, San Diego, CA 92182, USA e Fuller Evolutionary Biology Program, Cornell Lab of Ornithology, Cornell University, 159 Sapsucker Woods Road, Ithaca, NY 14950, USA f Department of Biology, University of Miami, 1301 Memorial Drive, Coral Gables, FL 33146, USA g Universidad San Francisco de Quito, USFQ, Colegio de Ciencias Biológicas y Ambientales, y Extensión Galápagos, Campus Cumbayá, Casilla Postal 17-1200-841, Quito, Ecuador article info abstract Article history: The New World sparrows (Emberizidae) are among the best known of songbird groups and have long- Received 6 November 2013 been recognized as one of the prominent components of the New World nine-primaried oscine assem- Revised 16 April 2014 blage. Despite receiving much attention from taxonomists over the years, and only recently using molec- Accepted 21 April 2014 ular methods, was a ‘‘core’’ sparrow clade established allowing the reconstruction of a phylogenetic Available online 30 April 2014 hypothesis that includes the full sampling of sparrow species diversity. -

Wildlife Habitat Plan
WILDLIFE HABITAT PLAN City of Novi, Michigan A QUALITY OF LIFE FOR THE 21ST CENTURY WILDLIFE HABITAT PLAN City of Novi, Michigan A QUALIlY OF LIFE FOR THE 21ST CENTURY JUNE 1993 Prepared By: Wildlife Management Services Brandon M. Rogers and Associates, P.C. JCK & Associates, Inc. ii ACKNOWLEDGEMENTS City Council Matthew C. Ouinn, Mayor Hugh C. Crawford, Mayor ProTem Nancy C. Cassis Carol A. Mason Tim Pope Robert D. Schmid Joseph G. Toth Planning Commission Kathleen S. McLallen, * Chairman John P. Balagna, Vice Chairman lodia Richards, Secretary Richard J. Clark Glen Bonaventura Laura J. lorenzo* Robert Mitzel* Timothy Gilberg Robert Taub City Manager Edward F. Kriewall Director of Planning and Community Development James R. Wahl Planning Consultant Team Wildlife Management Services - 640 Starkweather Plymouth, MI. 48170 Kevin Clark, Urban Wildlife Specialist Adrienne Kral, Wildlife Biologist Ashley long, Field Research Assistant Brandon M. Rogers and Associates, P.C. - 20490 Harper Ave. Harper Woods, MI. 48225 Unda C. lemke, RlA, ASLA JCK & Associates, Inc. - 45650 Grand River Ave. Novi, MI. 48374 Susan Tepatti, Water Resources Specialist * Participated with the Planning Consultant Team in developing the study. iii TABLE OF CONTENTS ACKNOWLEDGEMENTS iii PREFACE vii EXECUTIVE SUMMARY viii FRAGMENTATION OF NATURAL RESOURCES " ., , 1 Consequences ............................................ .. 1 Effects Of Forest Fragmentation 2 Edges 2 Reduction of habitat 2 SPECIES SAMPLING TECHNIQUES ................................ .. 3 Methodology 3 Survey Targets ............................................ ., 6 Ranking System ., , 7 Core Reserves . .. 7 Wildlife Movement Corridor .............................. .. 9 FIELD SURVEY RESULTS AND RECOMMENDATIONS , 9 Analysis Results ................................ .. 9 Core Reserves . .. 9 Findings and Recommendations , 9 WALLED LAKE CORE RESERVE - DETAILED STUDy.... .. .... .. .... .. 19 Results and Recommendations ............................... .. 21 GUIDELINES TO ECOLOGICAL LANDSCAPE PLANNING AND WILDLIFE CONSERVATION. -

The First Record of Common Ringed Plover (Charadrius Hiaticula) in British Columbia
The First Record of Common Ringed Plover (Charadrius hiaticula) in British Columbia. By Rick Toochin. Submitted: April 15, 2019. Introduction and Distribution The Common Ringed Plover (Charadrius hiaticula) is a widespread Old World shorebird species that is found breeding in the Arctic and subarctic regions from Greenland, Europe, east to Siberia (O’Brien et al. 2006). In North America, this species breeds on Baffin Island, eastern Ellesmere Island (Godfrey 1986). The Common Ringed Plover winters primarily from Western Europe, the Mediterranean Basin, throughout Africa, including Madagascar, and the Middle East (Hayman et al. 1986, O’Brien et al. 2006, Brazil 2009). There are three recognized subspecies of the Common Ringed Plover (Thies et al. 2018). Distinction between the subspecies is based on moult; with features changing clinally North to South, rather than East to West, making it impossible to draw a dividing line in Northwestern Europe (del Hoyo et al. 1996, Snow and Perrins 1998). The nominate subspecies of Common Ringed Plover is (C. h. hiaticula ) which breeds from southern Scandinavia to Great Britain, and northwestern France (Wiersma et al. 2019). This subspecies winters from Great Britain, south into Africa (del Hoyo et al. 1996, Snow and Perrins 1998). The second subspecies of the Common Ringed Plover is (C. h. tundrae) which is found breeding from northern Scandinavia, and northern Russia east to the Chukotskiy Peninsula, and is a casual breeder also in the northern Bering Sea region of Alaska on St Lawrence Island (Wiersma et al. 2019). This subspecies winters in the Caspian Sea region, and from Southwest Asia, south and east to South Africa (Wiersma et al. -

Nesting Habitat and Breeding Success of Fulica Atra in Tree Wetlands in Fez's Region, Central Morocco
J Anim Behav Biometeorol (2020) 8:282-287 ISSN 2318-1265 ORIGINAL ARTICLE Nesting habitat and breeding success of Fulica atra in tree wetlands in Fez’s region, central Morocco Wafae Squalli ▪ Ismail Mansouri ▪ Mohamed Dakki ▪ Fatima Fadil W Squalli (Corresponding author) ▪ I Mansouri ▪ F Fadil M Dakki Laboratory of Functional Ecology and Genie of Environment, Laboratory of Geo-biodiversity and Natural Heritage, Faculty of sciences and technology, USMBA, Fez, Morocco. Scientific Institute, Mohammed V University, Av. Ibn Battota, 10 BP 703, Rabat, Morocco. email: [email protected] Received: June 07, 2020 ▪ Accepted: July 15, 2020 ▪ Published Online: August 06, 2020 Abstract The current study was intended to investigate the in part, by interspecific (Fretwell and Lucas 1969; Jones 2001) breeding habitats and ecology of the Eurasian coot Fulica atra and intraspecific interactions (Morris 1989), climate contrast in Fez region Morocco. To achieve our goals, nests were (Martin 2001), and habitat degradation (Feary et al 2007). monitored in three wetlands Oued Al Jawahir river, Mahraz Understanding the dissimilarity between adaptive and and El Gaada dams. In addition, nesting vegetation and nest’s maladaptive animal use of habitat is needed for any dimensions were analysed to characterise the Eurasian coot conservation issue because animal use of inadequate habitat is nests. As results, 46 nests (74%) were found in Oued al counter to conservation drives (Case and Taper 2000). Jawahir, compared with 15 nests (24%) in Mahraz dam. In El Because species conservation worry often occurs in disturbed Gaada dam only 2 nests were built by the Eurasian coots. On habitats (Belaire et al 2014), patterns in habitat use in these the other hand, all nests were built on the riparian vegetation species may not always be revealing of the habitat conditions of the river and dams. -

Iucn Red Data List Information on Species Listed On, and Covered by Cms Appendices
UNEP/CMS/ScC-SC4/Doc.8/Rev.1/Annex 1 ANNEX 1 IUCN RED DATA LIST INFORMATION ON SPECIES LISTED ON, AND COVERED BY CMS APPENDICES Content General Information ................................................................................................................................................................................................................................ 2 Species in Appendix I ............................................................................................................................................................................................................................... 3 Mammalia ............................................................................................................................................................................................................................................ 4 Aves ...................................................................................................................................................................................................................................................... 7 Reptilia ............................................................................................................................................................................................................................................... 12 Pisces ................................................................................................................................................................................................................................................. -
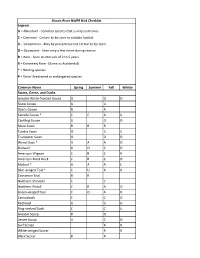
Common Name Spring Summer Fall Winter Greater White-Fronted Goose
Illinois River NWFR Bird Checklist Legend: A = Abundant - Common species that is very numerous C = Common - Certain to be seen in suitable habitat U = Uncommon - May be present but not certain to be seen O = Occasional - Seen only a few times during season R = Rare - Seen at intervals of 2 to 5 years X = Extremely Rare - (Same as Accidental) * = Nesting species # = State threatened or endangered species Common Name Spring Summer Fall Winter Swans, Geese, and Ducks Greater White-fronted Goose O O O Snow Goose U U Ross's Goose R R Canada Goose * C C A U Cackling Goose U O O Mute Swan R R R Tundra Swan O U U Trumpeter Swan O O O Wood Duck * A A A O Gadwall U O C O American Wigeon C R C R American Black Duck C R C O Mallard * A A A C Blue-winged Teal * C U A R Cinnamon Teal R R Northern Shoveler C C Northern Pintail C R A O Green-winged Teal C O A R Canvasback C C O Redhead U U O Ring-necked Duck C C U Greater Scaup R O Lesser Scaup A C O Surf Scoter R R White-winged Scoter R R Black Scoter R R Long-tailed Duck R R Bufflehead U U O Common Goldeneye U U U Hooded Merganser * C O C O Common Merganser C O C Red-breasted Merganser O O Ruddy Duck C R C U Upland Game Birds Ring-necked Pheasant O O O O Wild Turkey * O O O O Northern Bobwhite * U U U U Loons, Grebes, Pelicans, and Cormorants Red-throated Loon R Common Loon O O Pied-billed Grebe * C O U Horned Grebe U U Eared Grebe R R Western Grebe R American White Pelican A A O Double-crested Cormorant A O A O Bitterns, Herons, and Vultures American Bittern # R R R Least Bittern * # R R R Great Blue -

Ecuador: HARPY EAGLE & EAST ANDEAN FOOTHILLS EXTENSION
Tropical Birding Trip Report Ecuador: HARPY EAGLE & East Andean Foothills Extension (Jan-Feb 2021) A Tropical Birding custom extension Ecuador: HARPY EAGLE & EAST ANDEAN FOOTHILLS EXTENSION th nd 27 January - 2 February 2021 The main motivation for this custom extension was this Harpy Eagle. This was one of an unusually accessible nesting pair near the Amazonian town of Limoncocha that provided a worthy add-on to The Andes Introtour in northwest Ecuador that preceded this (Jose Illanes/Tropical Birding Tours). Guided by Jose Illanes Birds in the photos within this report are denoted in RED, all photos were taken by the Tropical Birding guide. 1 www.tropicalbirding.com +1-409-515-9110 [email protected] Tropical Birding Trip Report Ecuador: HARPY EAGLE & East Andean Foothills Extension (Jan-Feb 2021) INTRODUCTION This custom extension trip was set up for one person who simply could not get enough of Ecuador…John had just finished Ecuador: The Andes Introtour, in the northwest of the country, and also joined the High Andes Extension to that tour, which sampled the eastern highlands too. However, he was still missing vast chunks of this small country that is bursting with bird diversity. Most importantly, he was keen to get in on the latest “mega bird” in Ecuador, a very accessible Harpy Eagle nest, near a small Amazonian town, which had been hitting the local headlines and drawing the few birding tourists in the country at this time to come see it. With this in mind, TROPICAL BIRDING has been offering custom add-ons to all of our Ecuador offerings (for 2021 and 2022) to see this Harpy Eagle pair, with only three extra days needed to see it.