Silly Putty: Synthesizing a Polymer
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Living up to Life Special Stain Kit Modified Grocott's Methenamine
Living up to Life Special Stain Kit Modified Grocott’s Methenamine Silver Stain Catalog No: 38016SS12 Intended Use 16. Rinse slides briefly in deionized water. For In Vitro Diagnostic Use. For Laboratory Use. 17. Dehydrate slides in three changes of absolute alcohol. The reagents in this kit are intended for “In Vitro“ use only. The Modified Grocott’s Methenamine 18. Clear slides in two changes of xylene and mount in a xylene miscible medium. Silver Stain when used with appropriate histological protocols may be used for the demonstration of defined fungi and infectious agents such as Aspergillus sp., Pneumocystis Staining Protocol (Microwave) carinii and Cryptococcus neoformans in formalin fixed, paraffin embedded tissue sections. Exercise caution when using the microwave oven to heat any solution or reagent. The microwave must be properly ventilated to prevent the accumulation of fumes in the laboratory. Probable Mode of Action Microwave transparent Coplin jars and caps should be used during the staining process. The The mechanism of action of Modified Grocott’s Methenamine Silver Stain is based upon the caps should be loosely attached to prevent spills. Caps with ventilation holes also may be used. capacity of aldehyde groups to reduce cationic silver (Ag+) to metallic silver. Chromic acid is All microwave ovens should be used in accordance with the manufacturer’s instructions. The used to generate aldehyde groups by the oxidation of 1-2 glycol groups within polysaccharide procedures described here were performed using an Energy Beam Sciences H2250 laboratory rich tissue components, e.g. glycogen, mucin, reticulin and fungal cell walls. When cationic microwave. -

Accidental Discoveries
Accidental discoveries Part of the British Science Association’s British Science Week activity pack series. www.britishscienceweek.org British Science British British Science www.britishscienceweek.org 0 Accidental discoveries: Bakelite plastic Making silly putty More than one hundred years ago, electric wires were covered with ‘shellac’. It stopped people getting an electric shock if they touched the wire. It was very expensive because it was made from beetles that came from Asia. So, a man called Leo Baekeland decided to make a new type of covering. However, instead he discovered how to make the first plastic, called Bakelite. Plastics can be moulded into all kinds of shapes. They are used to make all sorts of things. You can have a go at making a bouncy plastic, called silly putty. You will need: 2 containers (such as plastic cups), a wooden stick (for This 1950s telephone is made from Bakelite stirring), some food colouring, PVA glue, borax solution (about 1 tablespoon of borax to a cup of water) What you do Put about one tablespoon of borax into one cup of water. Stir until it dissolves. Stir in two or three drops of food colouring. Put some PVA glue into the other container – just enough to cover the bottom. Pour some of the borax solution into the PVA glue. Stir the mixture until it makes a soft lump. By now the mixture should be joining together like putty. Take it out of the container and mould it into a ball in your hands. If it is still sticky, wash your hands with water and rinse the ball. -

Understanding Silly Putty, Snail Slime and Other Funny Fluids
IMA Annual Program Year Tutorial An Introduction to Funny (Complex) Fluids: Rheology, Modeling and Theorems September 12-13, 2009 Understanding silly putty, snail slime and other funny fluids Chris Macosko Department of Chemical Engineering and Materials Science NSF- MRSEC (National Science Foundation sponsored Materials Research Science and Engineering Center) IPRIME (Industrial Partnership for Research in Interfacial and Materials Engineering) What is rheology? ρειν (Greek) = to flow τα παντα ρει = every thing flows rheology = study of flow?, i.e. fluid mechanics? honey and mayonnaise honey and mayo stress= viscosity = f/area stress/rate rate of deformation rate of deformation What is rheology? ρειν (Greek) = to flow τα παντα ρει = every thing flows rheology = study of flow?, i.e. fluid mechanics? honey and mayo rubber band and silly putty viscosity modulus = f/area rate of deformation time of deformation 4 key rheological phenomena rheology = study of deformation of complex materials fluid mechanician: simple fluids complex flows materials chemist: complex fluids complex flows rheologist: complex fluids simple flows rheologist fits data to constitutive equations which - can be solved by fluid mechanician for complex flows - have a microstructural basis from: Rheology: Principles, Measurement and Applications, VCH/Wiley (1994). ad majorem Dei gloriam Goal: Understand Principles of Rheology: (stress, strain, constitutive equations) stress = f (deformation, time) Simplest constitutive relations: Newton’s Law: Hooke’s Law: dγ τ = η = ηγ τ = Gγ dt Key Rheological Phenomena • shear thinning (thickening) h ( g ) • time dependent modulus G(t) • normal stresses in shear N1 η η • extensional > shear stress u > k k 1 ELASTIC SOLID 1 2 The power of any spring L´ is in the same proportion with the tension thereof. -

Scientific Committee on Consumer Safety SCCS
SCCS/1249/09 Revision of 28 September 2010 Scientific Committee on Consumer Safety SCCS OPINION ON Boron compounds The SCCS adopted this opinion at its 7th plenary meeting of 22 June 2010 SCCS/1249/09 Opinion on boron compounds ___________________________________________________________________________________________ About the Scientific Committees Three independent non-food Scientific Committees provide the Commission with the scientific advice it needs when preparing policy and proposals relating to consumer safety, public health and the environment. The Committees also draw the Commission's attention to the new or emerging problems which may pose an actual or potential threat. They are: the Scientific Committee on Consumer Safety (SCCS), the Scientific Committee on Health and Environmental Risks (SCHER) and the Scientific Committee on Emerging and Newly Identified Health Risks (SCENIHR) and are made up of external experts. In addition, the Commission relies upon the work of the European Food Safety Authority (EFSA), the European Medicines Evaluation Agency (EMA), the European Centre for Disease prevention and Control (ECDC) and the European Chemicals Agency (ECHA). SCCS The Committee shall provide opinions on questions concerning all types of health and safety risks (notably chemical, biological, mechanical and other physical risks) of non-food consumer products (for example: cosmetic products and their ingredients, toys, textiles, clothing, personal care and household products such as detergents, etc.) and services (for example: tattooing, artificial sun tanning, etc.). Scientific Committee members Jürgen Angerer, Ulrike Bernauer, Claire Chambers, Qasim Chaudhry, Gisela Degen, Gerhard Eisenbrand, Thomas Platzek, Suresh Chandra Rastogi, Vera Rogiers, Christophe Rousselle, Tore Sanner, Kai Savolainen, Jacqueline Van Engelen, Maria Pilar Vinardell, Rosemary Waring, Ian R. -

THE CHEMISTRY of SILLY PUTTY Silly Putty Is an Odd Substance – It Can Be Slowly Stretched Out, but Snaps If Pulled Apart with Greater Force
THE CHEMISTRY OF SILLY PUTTY Silly putty is an odd substance – it can be slowly stretched out, but snaps if pulled apart with greater force. It can be molded into shape, but bounces if rolled into a ball. What’s behind these strange properties? Here’s a quick look at the chemical composition and explanation. WHAT IS IT MADE OF? HOW DOES IT WORK? The most important compound in silly putty The presence of PDMS alone, and its is polydimethylsiloxane (PDMS). This is the viscoelasticity, doesn’t fully explain how silly simplest member of the polymer family known putty behaves. Another ingredient, boric acid, as the silicones. also makes a telling contribution. PDMS is viscoelastic. This means that it acts The boric acid helps to create ‘crosslinks’ like a viscous liquid and flows over long time between adjacent polymer chains. These help scales. However, over short time scales (for to give silly putty its putty-like nature, and also example, being rolled into a ball and thrown at help explain its strange behaviour. a hard surface), its behaviour is elastic, and it will bounce back. R Si O O Si R – EXAMPLE BORON-MEDIATED CROSSLINK B Si Si Si (R represents the rest of the PDMS polymer chain) O O R Si O O Si R n A POLYDIMETHYLSILOXANE The polydimethylsiloxanes in silly putty end in Si-OH groups. The boric (solid filled atoms represent carbon; smaller outlined atoms represent hydrogen) acid reversibly reacts with these to form short-lived crosslinks between polymer chains. Slow deformation gives these crosslinks time to break and reform, allowing viscous flow, but rapid, forceful deformation does At high molecular weights, the flexible polymer chains become loosely not, so elastic behaviour is instead seen. -
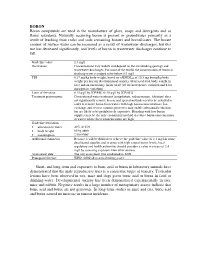
BORON Boron Compounds Are Used in the Manufacture of Glass, Soaps and Detergents and As Flame Retardants
BORON Boron compounds are used in the manufacture of glass, soaps and detergents and as flame retardants. Naturally occurring boron is present in groundwater primarily as a result of leaching from rocks and soils containing borates and borosilicates. The borate content of surface water can be increased as a result of wastewater discharges, but this use has decreased significantly, and levels of boron in wastewater discharges continue to fall. Guideline value 2.4 mg/l Occurrence Concentrations vary widely and depend on the surrounding geology and wastewater discharges. For most of the world, the concentration of boron in drinking-water is judged to be below 0.5 mg/l. TDI 0.17 mg/kg body weight, based on a BMDL 05 of 10.3 mg boron/kg body weight per day for developmental toxicity (decreased fetal body weight in rats) and an uncertainty factor of 60 (10 for interspecies variation and 6 for intraspecies variation) Limit of detection 0.15 µg/l by ICP/MS; 6–10 µg/l by ICP/AES Treatment performance Conventional water treatment (coagulation, sedimentation, filtration) does not significantly remove boron, and special methods need to be installed in order to remove boron from waters with high boron concentrations. Ion exchange and reverse osmosis processes may enable substantial reduction but are likely to be prohibitively expensive. Blending with low-boron supplies may be the only economical method to reduce boron concentrations in waters where these concentrations are high. Guideline derivation • allocation to water 40% of TDI • body weight 60 kg adult • consumption 2 litres/day Additional comments Because it will be difficult to achieve the guideline value of 2.4 mg/l in some desalinated supplies and in areas with high natural boron levels, local regulatory and health authorities should consider a value in excess of 2.4 mg/l by assessing exposure from other sources. -

Synthesis, Characterization and Fungicidal Effects of Some
Journal of Manmohan Memorial Institute of Health Sciences (JMMIHS) Synthesis, Characterization and Fungicidal Effects of some Organoborates Shaheen Akther1 Khanal DP2 Ullah SS3 Abstract Synthesis of benzoyl borate, phthasloyl borate and oxyl borate was done and characterized by infra red, ultraviolet and mass spectra. All newly synthesized and characterized organoborates were tested for their antifungal activity against pencillium that spoilt mostly celluloid materials stored in museum. All synthesized organoborates are unstable in room temperature and quickly degraded on exposure to air. Benzoyl borate found to be most active antifungal compound of the series against penicillium. The fungicidal activity increases in hydrophobicity and electron withdrawing nature of the benzene ring substituents. These compounds may be good fungicidal for the conservation of celluloid materials lying in the museum. Key words: Organoborates, Silver metaborate, Acyl chloride, spore, Antifungal. Introduction All organoborates are synthesized with the used of silver metaborate (AgBO2) and boric acid [1] with organic halides such as 3,5, dinitrobenzoyl chloride and p-nitrobenzoyl chloride. Since the silver atom in AgBO2 in photo activated state the reaction with organic halides having a polarized halogen atom will readily takes place with the formation of silver halides. Steric effect is an important factor in determining the course of reactions well as the stability of the product. It is observed that acyl chloride reacts more readily than sterically hindered pyrollium chloride [2]. The reaction of silver metaborate with organic halides may be ascribed to an ionic mechanism. Indeed acyllium [3] ion have been recognized as possible intermediate in acid catalyzed etherification in Friedel- Crafts reactions through the universality of such mechanism in doubt and the ion is assumed to resonate between the below structures: R – C O+ R – C+ O The presence of CO group polarizes the carbon halogen bond to great extent. -

Fourteen K-12 Outreach Activities for Materials Scientists and Engineers
FOURTEEN K-12 OUTREACH ACTIVITIES FOR MATERIALS SCIENTISTS AND ENGINEERS Foreward The activities described in this document were performed by volunteers from ASM International chapters as part of our volunteer leader training in 2007. The event took place in an elegant room with carpet, drapes, and wallpaper so heat treating was done in advance. A similar approach could be used to make the “heat treating” activities safe for younger students. Please take the utmost care to consider safety aspects as you plan your outreach event. Safety glasses that fit over corrective lenses are needed for many of these activities, and you may have trouble finding them small enough to fit young students. Many of the activities were gleaned from the ASM Materials Education Foundation’s “Teachers Camp” curriculum and have been proven in classrooms. I’m particularly grateful to Debbie Goodwin (Master Teacher) and Matt Perricone for their contributions to this compilation, and to Dr. Kathy Hayrynen of the Detroit Chapter of ASM International for her ongoing contributions to ASM’s outreach projects at many levels. All of the experimental descriptions in this compilation are either free of copyright protection, or have been used in accordance with the use restrictions of the original source. Original sources for each activity have been noted in the appendices. Commercial sources for some supplies have been mentioned as a convenience, and are not an endorsement of the particular company by ASM International, The ASM Foundation, or me. I hope you’ll have as much fun sharing our profession with students and teachers as I do. -

Results of Long-Term Carcinogenicity Bioassay on Vinyl Acetate Monomer in Sprague-Dawley Rats
Results of Long-Term Carcinogenicity Bioassay on Vinyl Acetate Monomer in Sprague-Dawley Rats FRANCO MINARDI, FIORELLA BELPOGGI, MORANDO SOFFRITTI, ADRIANO CILIBERTI, MICHELINA LAURIOLA, ELISA CATTIN, AND CESARE MALTONI† Cancer Research Center, European Ramazzini Foundation for Oncology and Environmental Sciences, Bologna, Italy ABSTRACT: Vinyl acetate monomer (VAM) was administered in drinking water supplied ad libitum at doses of 5,000, 1,000, and 0 ppm (v/v) to 17-week-old Sprague-Dawley rats (breeders) and to 12-day embryos (offspring). Treatment lasted for 104 weeks; thereafter, animals were kept under control conditions until spontaneous death. VAM was found to cause an increase in total malig- nant tumors and in carcinomas and/or precursor lesions of the oral cavity, lips, tongue, esophagus, and forestomach. Based on these data, VAM must be con- sidered a multipotent carcinogen. KEYWORDS: vinyl acetate monomer; carcinogenicity; long-term bioassay; rat INTRODUCTION Vinyl acetate monomer (VAM) is an important compound in the plastics industry. VAM (C 4H6O2) has a molecular weight of 86.09. Industrial production of VAM started in the United States in 1928.1 VAM is produced mainly by two processes: (1) In a process used since the 1920s, acetylene and acetic acid are reacted in the vapor phase over a catalyst bed,2 (2) In another process, largely used since the 1970s, eth- ylene is reacted with acetic acid in the presence of oxygen.3 The world production of VAM is over 2.5 million tons per year.4 The only commercial use of VAM is in the production of polymers (polyvinyl ac- etate, polyvinyl alcohol, polyvinyl acetals) and copolymers (ethylene-vinyl acetate and polyvinyl-acetate chloride).3 Polyvinyl acetate is mainly used in adhesives for paper, wood, glass, metals, and porcelain. -

Sept. 4, 1962. MICHIJIRO AKABOSH ETAL 3,052,610 CONCENTRATION of ACETIC ACID Filed May 19, 1960
Sept. 4, 1962. MICHIJIRO AKABOSH ETAL 3,052,610 CONCENTRATION OF ACETIC ACID Filed May 19, 1960 INVENTORS MCHIJIRO AKABOSH KK UJ U RAGAM: BY KENSUKE O KUMA AttoRNEY 3,052,610 United States Patent Office Patented Sept. 4, 1962 2 1. acetate. Some of the vinyl acetate from the upper phase 3,052,610 is returned to the top of the distillation zone as reflux, CONCENTRATION OF ACETIC ACD in accordance with conventional distillation technique, Michijiro Akaboshi, Toyonaka City, and Kikuji Uragaini and the remainder is withdrawn as product. and Kensuke Okema, Toyama City, Japan, assignors to Particularly suitable as a source of vinyl acetate for Kurashiki Rayon Co., Ltd., Okayama Prefecture, the distillation operation referred to above is the vinyl Japan, a corporation of Japan acetate-acetic acid mixture which is produced in the syn Fied May 19, 1960, Ser. No. 30,384 thesis of vinyl acetate from acetylene and acetic acid. 6 Claims. (C. 202-42) Prior to its use for the production of polyvinyl acetate This invention relates to the concentration of aqueous O by polymerization, the vinyl acetate must be separated Solutions of acetic acid and is more particularly concerned from the acetic acid. In accordance with the invention, With a process for concentrating aqueous acetic acid solu this operation is combined with the acetic acid dehydrat tions which is effectively integrated with the manufac ing and concentrating operation so that two normally ture of polyvinyl alcohol. independent processing steps are effectively and efficiently In accordance with conventional practice, polyvinyl 5 combined into a single operation which produces sub alcohol is formed by the saponification or "alcoholysis' stantially pure vinyl acetate suitable for the production of polyvinyl acetate which, in turn, is formed by the poly of polyvinyl acetate, and an effectively concentrated or merization of vinyl acetate. -

The Effects of Borax on Milk Yield and Selected Metabolic Parameters in Austrian Simmental (Fleckvieh) Cows
Veterinarni Medicina, 60, 2015 (4): 175–180 Original Paper doi: 10.17221/8104-VETMED The effects of borax on milk yield and selected metabolic parameters in Austrian Simmental (Fleckvieh) cows M. Kabu, C. Uyarlar Faculty of Veterinary Medicine, Afyon Kocatepe University, Afyonkarahisar, Turkey ABSTRACT: This study was conducted to determine the effects of orally administered borax on milk yield and on several blood variables related to metabolism in early lactation in Austrian Simmental cows (Fleckvieh). Twenty primiparous cows were selected at parturition and then assigned to one of two groups, the control group or the borax group. The study lasted for four weeks. Borax was administered orally at 0.2 mg/kg/day (Boron group) to all treatment cows shortly after the noon milking, whereas cows in the control group were not treated. All cows consumed the same diet. All feeds in the diet were analysed for crude cellulose, protein, ether extract, ash, and dry matter according to the Weende Analysis Systems, in addition to ADF and NDF, according to Van Soest. Blood samples were collected from all cows via the vena jugularis on lactation Days 0, 7, 15, 21 and 28 and ana- lysed for the following: serum boron (B), non-esterified fatty acids (NEFA), beta-hydroxybutyric acid (BHBA), total cholesterol (TChol), high density lipids (HDL), total protein (TP), blood urea nitrogen (BUN), albumin (ALB), creatine (CRE), uric acid (UA), glucose (GLU), aspartate aminotransferase (AST), alanine aminotrans- ferase (ALT) and gamma-glutamyl transpeptidase (GGT) concentrations. Serum B concentration was higher in the borax group than in the control group at Weeks 1, 2, 3, and 4 of the experiment. -

Gak and Non-Newtonian Fluid
1) A polymer consists of long chained molecules that are bound together. 2) Non-Newtonian fluids are those that have viscosities that change with conditions of stress or time. Background Gak – Glue consists of long chained molecules that are very loosely tied together, which explains why it has a higher viscosity than water. When mixed with Borax, the molecules are “tangled” and a chemical reaction takes place. The tangled chains are polymers which is what plastics are and other man made substances such as nylon. http://www.petervaldivia.com/technology/plastics/i mage/polymer.gif&imgrefurl=http://www.peterval divia.com/technology/plastics/index A Non-Newtonian fluid is one that is not characterized by one viscosity. Such fluids have viscosities that change as a function of stress, time or both. A mixture of cornstarch and water flows when little stress is applied, yet acts like a solid when a lot of stress is applied – for example, hitting the surface with your hand or even jumping quickly on it. Uses of such fluids may be in body armor. There are other non-Newtonian fluids that have the opposite reaction to stress. Fluids such as ketchup become less viscous when stress is applied (hence hitting the bottle of ketchup allows the ketchup to flow out). Cornstarch and water mixture Cornstarch and water mixture on a speaker http://upload.wikimedia.org/wikipedia/common s/f/f0/Non-Newtonian_fluid.PNG Materials Demonstration – polymers Gak (per group) Various plastic containers. Dixie cup for mixing Wooden stirrer Small plastic ziplock bag for storing Demonstration – Viscosity Borax/water mixture Glue/water mixture Plastic board Food Coloring if desired Various liquids of different viscosity to drip on slanted board and watch run them run off Non Newtonian fluid (per group) Ketchup in a glass bottle Dixie cup for mixing Wooden stirrer Demonstration – Pool of Small plastic ziplock bag for storing Cornstarch/water Cornstarch Water Kiddie pool of cornstarch/water.