Commercial Formulary
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

2021 National Formulary 3Rd Quarter Edition
2021 National Formulary 3rd Quarter Edition Last Revised: 08/18/2021 Version 2021Q3c Table of Contents OVERVIEW 4 CARDIOVASCULAR (HEART) DRUGS 14 Alpha & Beta Blockers 14 COVERAGE LIMITATION 4 Antihypertensive Combinations 14 Calcium Channel Blockers (CCBs) 14 COMPOUNDED DRUGS 4 ACE Inhibitors without & with Diuretics 15 DRUG PLACEMENT DETERMINATION 4 ACE Inhibitors / CCB Combinations 15 ARBs without & with Diuretics 15 PREFERRED BRAND PRODUCTS 5 ARB Combinations 15 Naprilysin Inhibitors 15 GENERIC SUBSTITUTION 5 Diuretics 15 Renin Inhibtors 16 SINGLE & DUAL SOURCE GENERICS 5 Antiarrhythmics/Anti-Ischemic 16 Cardiac Glycosides 16 PRIOR AUTHORIZATIONS, STEP EDITS & QTY LIMITS 6 Vasodilators, Coronary, Nitrates/Vasodilators, Sympatholytics 16 EXCLUDED DRUGS 6 Other Drugs 16 NON-LISTED DRUGS & DRUG CATEGORIES 7 ANTIHYPERLIPIDEMIC (CHOLESTEROL) DRUGS 17 Statins & Statin/CCB Combinations 17 FORMULARY MODIFICATIONS & CHANGES 7 Bile Acid Sequestrants, Liver Drugs 17 Fibrates 17 BIOSIMILARS 7 ACL Inhibitors 17 Other Drugs 17 MAJOR CHANGES TO THE PDL 7 PANCREATIC DRUGS 18 ANTIBIOTICS 8 Penicillins & Cephalosporins 8 KIDNEY & URINARY / UROLOGICAL DRUGS 18 Tetracyclines 8 Benign Prostate Hyperplasia 18 Macrolides & Clindamycins 8 Urologic Drugs / Other Drugs 18 Sulfonamides, Sulfones & Ketolides 8 Erectile Dysfunction Drugs 18 Quinolones 8 Gout Drugs – Purine Inhibitors 19 Miscellaneous Antibiotics 8 Urinary Ph Modifiers 19 Potassium & Electrolytes 19 ANTI-VIRALS 9 Phosphorus/Calcium/Electrolyte Depleters 19 General Antivirals 9 HIV Antiviral Drugs 9 OSTEOPOROSIS (BONE) DRUGS 20 HIV Pre-Exposure Propylaxis Drugs 9 ANTI-INFLAMMATORY / ANALGESIC (PAIN) DRUGS 20 ANTI-INFECTIVES 10 Anti-Inflammatory Drugs (NSAIDS) 20 Anaerobic Anti-Infectives 10 COX-II Drugs 21 Antiparasitics 10 Analgesics, Narcotics (Opioids) 21 Antimalarials & Antiprotozoals 10 Analgesics, Salicylates, Non-Salicylates, Other 21 Antihelmintic Drugs 10 CENTRAL NERVOUS SYSTEM DRUGS 22 ANTIEMETICS 10 Anti-Anxiety Drugs (Benzodiazepines) 22 Sedative/Sleeping Drugs 22 NEUROLOGIC DRUGS 11 A.D.D. -
3-Local-Anesthetics.Pdf
Overview • Local anesthetics produce a transient and reversible loss of sensation (analgesia) in a circumscribed region of the body without loss of consciousness. • Normally, the process is completely reversible. • Local anesthetics are generally classified as either esters or amides and are usually linked to: – a lipophilic aromatic group – to a hydrophilic, ionizable tertiary (sometimes secondary) amine. • Most are weak bases with pKa ( 8 – 9), and at physiologic pH they are primarily in the charged, cationic form. • The potency of local anesthetics is positively correlated with their lipid solubility, which may vary 16-fold, and negatively correlated with their molecular size. • These anesthetics are selected for use on the basis of: 1. the duration of drug action • Short: 20 min • Intermediate: 1—1.5 hrs • Long: 2—4 hrs 2. effectiveness at the administration site 3. potential for toxicity Mechanism of action Local anesthetics act by blocking sodium channels and the conduction of action potentials along sensory nerves. • Blockade is voltage dependent and time dependent. a. At rest, the voltage-dependent sodium (Na+) channels of sensory nerves are in the resting (closed) state. • Following the action potential the Na+ channel becomes active (open) and then converts to an inactive (closed) state that is insensitive to depolarization. • Following repolarization of the plasma membrane there is a slow reversion of channels from the inactive to the resting state, which can again be activated by depolarization. • During excitation the cationic charged form of local anesthetics interacts preferentially with the inactivated state of the Na+ channels on the inner aspect of the sodium channel to block sodium current and increase the threshold for excitation. -

AHFS Pharmacologic-Therapeutic Classification System
AHFS Pharmacologic-Therapeutic Classification System Abacavir 48:24 - Mucolytic Agents - 382638 8:18.08.20 - HIV Nucleoside and Nucleotide Reverse Acitretin 84:92 - Skin and Mucous Membrane Agents, Abaloparatide 68:24.08 - Parathyroid Agents - 317036 Aclidinium Abatacept 12:08.08 - Antimuscarinics/Antispasmodics - 313022 92:36 - Disease-modifying Antirheumatic Drugs - Acrivastine 92:20 - Immunomodulatory Agents - 306003 4:08 - Second Generation Antihistamines - 394040 Abciximab 48:04.08 - Second Generation Antihistamines - 394040 20:12.18 - Platelet-aggregation Inhibitors - 395014 Acyclovir Abemaciclib 8:18.32 - Nucleosides and Nucleotides - 381045 10:00 - Antineoplastic Agents - 317058 84:04.06 - Antivirals - 381036 Abiraterone Adalimumab; -adaz 10:00 - Antineoplastic Agents - 311027 92:36 - Disease-modifying Antirheumatic Drugs - AbobotulinumtoxinA 56:92 - GI Drugs, Miscellaneous - 302046 92:20 - Immunomodulatory Agents - 302046 92:92 - Other Miscellaneous Therapeutic Agents - 12:20.92 - Skeletal Muscle Relaxants, Miscellaneous - Adapalene 84:92 - Skin and Mucous Membrane Agents, Acalabrutinib 10:00 - Antineoplastic Agents - 317059 Adefovir Acamprosate 8:18.32 - Nucleosides and Nucleotides - 302036 28:92 - Central Nervous System Agents, Adenosine 24:04.04.24 - Class IV Antiarrhythmics - 304010 Acarbose Adenovirus Vaccine Live Oral 68:20.02 - alpha-Glucosidase Inhibitors - 396015 80:12 - Vaccines - 315016 Acebutolol Ado-Trastuzumab 24:24 - beta-Adrenergic Blocking Agents - 387003 10:00 - Antineoplastic Agents - 313041 12:16.08.08 - Selective -

List of Union Reference Dates A
Active substance name (INN) EU DLP BfArM / BAH DLP yearly PSUR 6-month-PSUR yearly PSUR bis DLP (List of Union PSUR Submission Reference Dates and Frequency (List of Union Frequency of Reference Dates and submission of Periodic Frequency of submission of Safety Update Reports, Periodic Safety Update 30 Nov. 2012) Reports, 30 Nov. -
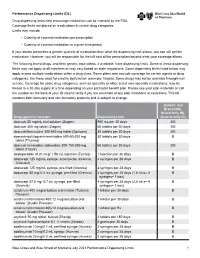
Performance Drug List Dispensing Limits
Performance Dispensing Limits (DL) Drug dispensing limits help encourage medication use as intended by the FDA. Coverage limits are placed on medications in certain drug categories. Limits may include: • Quantity of covered medication per prescription • Quantity of covered medication in a given time period If your doctor prescribes a greater quantity of medication than what the dispensing limit allows, you can still get the medication. However, you will be responsible for the full cost of the prescription beyond what your coverage allows. The following brand drugs, and their generic equivalents, if available, have dispensing limits. Some of these dispensing limits may not apply to all members or may vary based on state regulations. Some dispensing limits listed below may apply across multiple medications within a drug class. Some plans may exclude coverage for certain agents or drug categories, like those used for erectile dysfunction (example: Viagra). Some drugs may not be available through mail service. Coverage for some drug categories, such as specialty or other select non‑specialty medications, may be limited to a 30‑day supply at a time depending on your particular benefit plan. Please see your plan materials or call the number on the back of your ID card to verify if you are uncertain of any plan limitations or exclusions. This list contains both formulary and non‑formulary products and is subject to change. Generic and Brand (BG), Brand Only (B), Drug (generic) strength Dispensing Limit Generic only (G) abacavir 20 mg/mL oral -

WHO Model List of Essential Medicines
WHO Model List of Essential Medicines 15th list, March 2007 Status of this document This is a reprint of the text on the WHO Medicines web site http://www.who.int/medicines/publications/essentialmedicines/en/index.html 15th edition Essential Medicines WHO Model List (revised March 2007) Explanatory Notes The core list presents a list of minimum medicine needs for a basic health care system, listing the most efficacious, safe and cost‐effective medicines for priority conditions. Priority conditions are selected on the basis of current and estimated future public health relevance, and potential for safe and cost‐effective treatment. The complementary list presents essential medicines for priority diseases, for which specialized diagnostic or monitoring facilities, and/or specialist medical care, and/or specialist training are needed. In case of doubt medicines may also be listed as complementary on the basis of consistent higher costs or less attractive cost‐effectiveness in a variety of settings. The square box symbol () is primarily intended to indicate similar clinical performance within a pharmacological class. The listed medicine should be the example of the class for which there is the best evidence for effectiveness and safety. In some cases, this may be the first medicine that is licensed for marketing; in other instances, subsequently licensed compounds may be safer or more effective. Where there is no difference in terms of efficacy and safety data, the listed medicine should be the one that is generally available at the lowest price, based on international drug price information sources. Therapeutic equivalence is only indicated on the basis of reviews of efficacy and safety and when consistent with WHO clinical guidelines. -

WO 2016/133483 Al 25 August 2016 (25.08.2016) P O P C T
(12) INTERNATIONAL APPLICATION PUBLISHED UNDER THE PATENT COOPERATION TREATY (PCT) (19) World Intellectual Property Organization I International Bureau (10) International Publication Number (43) International Publication Date WO 2016/133483 Al 25 August 2016 (25.08.2016) P O P C T (51) International Patent Classification: SHENIA, Iaroslav Viktorovych [UA/UA]; Feodosiyskyy A61L 15/44 (2006.01) A61L 26/00 (2006.01) lane, 14-a, kv. 65, Kyiv, 03028 (UA). A61L 15/54 (2006.01) (74) Agent: BRAGARNYK, Oleksandr Mykolayovych; str. (21) International Application Number: Lomonosova, 60/5-43, Kyiv, 03189 (UA). PCT/UA20 16/0000 19 (81) Designated States (unless otherwise indicated, for every (22) International Filing Date: kind of national protection available): AE, AG, AL, AM, 15 February 2016 (15.02.2016) AO, AT, AU, AZ, BA, BB, BG, BH, BN, BR, BW, BY, BZ, CA, CH, CL, CN, CO, CR, CU, CZ, DE, DK, DM, (25) Filing Language: English DO, DZ, EC, EE, EG, ES, FI, GB, GD, GE, GH, GM, GT, (26) Publication Language: English HN, HR, HU, ID, IL, IN, IR, IS, JP, KE, KG, KN, KP, KR, KZ, LA, LC, LK, LR, LS, LU, LY, MA, MD, ME, MG, (30) Priority Data: MK, MN, MW, MX, MY, MZ, NA, NG, NI, NO, NZ, OM, a 2015 01285 16 February 2015 (16.02.2015) UA PA, PE, PG, PH, PL, PT, QA, RO, RS, RU, RW, SA, SC, u 2015 01288 16 February 2015 (16.02.2015) UA SD, SE, SG, SK, SL, SM, ST, SV, SY, TH, TJ, TM, TN, (72) Inventors; and TR, TT, TZ, UA, UG, US, UZ, VC, VN, ZA, ZM, ZW. -

Gonadotropin Therapy in Assisted Reproduction: an Evolutionary Perspective from Biologics to Biotech
REVIEW Gonadotropin therapy in assisted reproduction: an evolutionary perspective from biologics to biotech Roge´rio de Barros F. Lea˜ o, Sandro C. Esteves Andrology & Human Reproduction Clinic (ANDROFERT), Referral Center for Male Reproduction, Campinas/SP, Brazil. Gonadotropin therapy plays an integral role in ovarian stimulation for infertility treatments. Efforts have been made over the last century to improve gonadotropin preparations. Undoubtedly, current gonadotropins have better quality and safety profiles as well as clinical efficacy than earlier ones. A major achievement has been introducing recombinant technology in the manufacturing processes for follicle-stimulating hormone, luteinizing hormone, and human chorionic gonadotropin. Recombinant gonadotropins are purer than urine- derived gonadotropins, and incorporating vial filling by mass virtually eliminated batch-to-batch variations and enabled accurate dosing. Recombinant and fill-by-mass technologies have been the driving forces for launching of prefilled pen devices for more patient-friendly ovarian stimulation. The most recent developments include the fixed combination of follitropin alfa + lutropin alfa, long-acting FSH gonadotropin, and a new family of prefilled pen injector devices for administration of recombinant gonadotropins. The next step would be the production of orally bioactive molecules with selective follicle-stimulating hormone and luteinizing hormone activity. KEYWORDS: Gonadotropins; Ovulation Induction; Assisted Reproductive Techniques; Systematic Review. -

Prolonged Release of the Local Anesthetic Butamben for Potential Use in Pain Management
Pharmacology & Pharmacy, 2012, 3, 291-294 291 http://dx.doi.org/10.4236/pp.2012.33038 Published Online July 2012 (http://www.SciRP.org/journal/pp) Prolonged Release of the Local Anesthetic Butamben for Potential Use in Pain Management Ashish Rastogi1,2, Salomon Stavchansky2, Phillip D. Bowman1* 1US Army Institute of Surgical Research, Fort Sam Houston, USA; 2Division of Pharmaceutics, College of Pharmacy, The Univer- sity of Texas at Austin, Austin, USA. Email: [email protected], *[email protected] Received March 20th, 2012; revised April 29th, 2012; accepted May 11th, 2012 ABSTRACT Continuous delivery of local anesthetics might be useful for management of localized and chronic pain. Controlled re- lease injectable anesthetics have been developed but they can deliver the drug for only few days and the release is not zero-order. A drug delivery system (DDS) consisting of a perforated reservoir for drug containment and release and its potential for management of chronic pain is described. Proof of principle is detailed for long-term zero order delivery of butamben. In this study, the DDS was a polyimide tube with a 0.20 mm hole and butamben release was evaluated in vitro. It is envisioned that the DDS could be implanted in proximity to a nerve, enervating the pain source, for long-term control of chronic pain. Keywords: Controlled Release; Prolonged Drug Delivery; Pain Management; Butamben 1. Introduction operated, and not suitable for everyone [9]. Persistent cerebrospinal fluid leak during its insertion is also a Long-term drug therapy for pain management is chal- known complication [10]. -

15-CETY-040, Cetecaine Gel Sell Sheet FA No Crops
Cetacaine® TOPICAL ANESTHETIC GEL (Benzocaine 14.0%, Butamben 2.0%, Tetracaine Hydrochloride 2.0%) Proven more effective than benzocaine alone1 Cetacaine® Topical Anesthetic Gel is a fast-acting, long-lasting prescription topical anesthetic. Applied directly to the mucous membrane, Cetacaine is primarily used to control pain and ease discomfort at the application site. The protective pump-top jar controls the amount of Cetacaine dispensed while keeping the remaining contents safe from cross contamination. The outside lid helps to keep the pump surface clean and intact. Please see the Brief Summary of the Prescribing Information on the reverse side. You are encouraged to report negative side effects of prescription drugs to the FDA. Visit www.fda.gov/medwatch, or call 1-800-FDA-1088. Cetacaine Gel is indicated for anesthesia of all Description Size Item No. accessible mucous membrane except the eyes. Cetacaine Topical Anesthetic Gel 32 g 0217 Cetacaine is not for injection. Features & Benefits • Onset of action: Approximately 30 seconds* • Applies evenly and consistently, tissue need not be dried • Duration of action: 30-60 minutes* • Reacts with body temperature to melt and absorb quickly into tissue • Controls pain and eases discomfort at the application site • Made in USA • Pleasant strawberry flavor • Study shows Cetacaine’s triple formula is more effective than • Lubricating qualities Benzocaine alone Important Safety Information • On rare occasions, methemoglobinemia has been reported in • The most common adverse reaction caused by local anesthetics is connection with the use of benzocaine-containing products. contact dermatitis characterized by erythema and pruritus that If a patient becomes cyanotic, treat appropriately to counteract may progress to vesiculation and oozing. -

Infertility Therapy Reference Number: CP.CPA.261 Effective Date: 11.16.16 Last Review Date: 11.17 Line of Business: Medicaid – Medi-Cal Revision Log
Clinical Policy: Infertility Therapy Reference Number: CP.CPA.261 Effective Date: 11.16.16 Last Review Date: 11.17 Line of Business: Medicaid – Medi-Cal Revision Log See Important Reminder at the end of this policy for important regulatory and legal information. Description The following are gonadotropins requiring prior authorization: Menotropins (Menopur®), Follitropin alpha, recombinant (Gonal-F® RFF), Follitropin beta, recombinant (Follistim®-AQ), Urofollitropin (Bravelle®), Choriogonadotropin alfa (Ovidrel®), Human chorionic gonadotropin (Novarel®, Pregnyl®), Ganirelex acetate, Cetrorelix (Cetrotide®). FDA approved indication Menopur is indicated for development of multiple follicles and pregnancy in ovulatory women as part of an Assisted Reproductive Technology (ART) cycle. Gonal-F RFF is indicated: • For induction of ovulation and pregnancy in oligo-anovulatory women in whom the cause of infertility is functional and not due to primary ovarian failure. • For development of multiple follicles in ovulatory women as part of an Assisted Reproductive Technology (ART) cycle. Follistim AQ is indicated: • In women for: Induction of ovulation and pregnancy in anovulatory infertile women in whom the cause of infertility is functional and not due to primary ovarian failure. • In women for: Pregnancy in normal ovulatory women undergoing controlled ovarian stimulation as part of an In Vitro Fertilization (IVF) or Intracytoplasmic Sperm Injection (ICSI) cycle. • In men for: Induction of spermatogenesis in men with primary and secondary hypogonadotropic hypogonadism (HH) in whom the cause of infertility is not due to primary testicular failure. Bravelle is indicated: • For induction of ovulation in women who have previously received pituitary suppression. • For development of multiple follicles as part of an Assisted Reproductive Technology (ART) cycle in ovulatory women who have previously received pituitary suppression. -

Adverse Health Effects of Heavy Metals in Children
TRAINING FOR HEALTH CARE PROVIDERS [Date …Place …Event …Sponsor …Organizer] ADVERSE HEALTH EFFECTS OF HEAVY METALS IN CHILDREN Children's Health and the Environment WHO Training Package for the Health Sector World Health Organization www.who.int/ceh October 2011 1 <<NOTE TO USER: Please add details of the date, time, place and sponsorship of the meeting for which you are using this presentation in the space indicated.>> <<NOTE TO USER: This is a large set of slides from which the presenter should select the most relevant ones to use in a specific presentation. These slides cover many facets of the problem. Present only those slides that apply most directly to the local situation in the region. Please replace the examples, data, pictures and case studies with ones that are relevant to your situation.>> <<NOTE TO USER: This slide set discusses routes of exposure, adverse health effects and case studies from environmental exposure to heavy metals, other than lead and mercury, please go to the modules on lead and mercury for more information on those. Please refer to other modules (e.g. water, neurodevelopment, biomonitoring, environmental and developmental origins of disease) for complementary information>> Children and heavy metals LEARNING OBJECTIVES To define the spectrum of heavy metals (others than lead and mercury) with adverse effects on human health To describe the epidemiology of adverse effects of heavy metals (Arsenic, Cadmium, Copper and Thallium) in children To describe sources and routes of exposure of children to those heavy metals To understand the mechanism and illustrate the clinical effects of heavy metals’ toxicity To discuss the strategy of prevention of heavy metals’ adverse effects 2 The scope of this module is to provide an overview of the public health impact, adverse health effects, epidemiology, mechanism of action and prevention of heavy metals (other than lead and mercury) toxicity in children.