As Yet There Was No Field Shrub on Earth and No Grass of The
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

ISFC Annual Report 1999
1999 Salmon, Sea Trout . 3 Location Map for Awards Presentation in Doyle Burlington Brown Trout (Lake) . 4 Brown Trout (River) . 5 Bream . 6 Pike (Lake), Pike (River) . 8 Carp . 10 Eel, Roach/Bream Hybrid . 11 Rudd/Bream Hybrid, Perch . .12 Tench . 13 Bass . 14 Coalfish, Cod, Conger Eel, Dab, Greater Spotted Dogfish . 15 Lesser Spotted Dogfish, Spur Dogfish . 16 Flounder, Garfish, Grey Gurnard . 17 Red Gurnard, Tub Gurnard, Ling . 18 Mackerel . 19 Grey Mullet, Plaice . 20 ONTENTS Pollack, Pouting . 21 Blonde Ray, Homelyn Ray, Painted Ray . 22 Sting Ray, Three Bearded Rockling, Twaite Shad . 24 C Blue Shark . 25 Tope, Torsk, Ballan Wrasse, Cuckoo Wrasse . 26 New Records, Ten Species Award, Ten Pin Awards, Special Award for Juveniles, The Minister’s Award, . .27 Revised Specimen Weight/New Class, Special Notice, Limitation on Number of Claims, Exclusion from Specimen Status, Weighing of Fish, Metrification . 28 Common Skate, Captors Addresses, Distribution of Specimen Awards . .29 Acknowledgements, Presentation of Awards 1998, Fund Raising . 30 Accounts, Donations . 31 Use of the information contained in this report for press articles Balance Sheet . 32 and publicity is encouraged. It may be quoted without charge, Irish Record Fish Listing . 33 provided the source is acknowledged. Schedule of Specimen Weights (Revised) . 35 The report is copyright and prior permission to reproduce the Rules . 37 data for any other purpose other than reasonable review or Weighing Scale Certification – List of Centres . .40 analysis must be obtained in writing from the Irish Specimen Fish “Read it Carefully” by Des Brennan . 42 Committee. “Maybe we’ll stay at home this year!” by Derek Evans . -

Flood Analysis of the Clare River Catchment Considering Traditional Factors and Climate Change
Flood Analysis of the Clare River Catchment Considering Traditional Factors and Climate Change AUTHOR Pierce Faherty G00073632 A Thesis Submitted in Part Fulfilment for the Award of M.Sc. Environmental Systems, at the College of Engineering, Galway Mayo Institute of Technology, Ireland Submitted to the Galway Mayo Institute of Technology, September 2010 .... ITUTE Of TECHNOLOGY DECLARATION OF ORIGINALITY September 2010 The substance of this thesis is the original work of the author and due reference and acknowledgement has been made, when necessary, to the work of others. No part of this thesis has been accepted for any degree and is not concurrently submitted for any other award. I declare that this thesis is my original work except where otherwise stated. Pierce Faherty Sean Moloney Date: 1 7 - 01" 10__ Abstract The main objective of this thesis on flooding was to produce a detailed report on flooding with specific reference to the Clare River catchment. Past flooding in the Clare River catchment was assessed with specific reference to the November 2009 flood event. A Geographic Information System was used to produce a graphical representation of the spatial distribution of the November 2009 flood. Flood risk is prominent within the Clare River catchment especially in the region of Claregalway. The recent flooding events of November 2009 produced significant fluvial flooding from the Clare River. This resulted in considerable flood damage to property. There were also hidden costs such as the economic impact of the closing of the N17 until floodwater subsided. Land use and channel conditions are traditional factors that have long been recognised for their effect on flooding processes. -
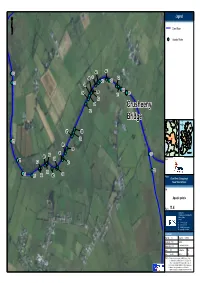
66C502f95e5b4c37b6f248c34de
1616 Legend ClareClare RiverRiver AquaticAquatic PointsPoints 4444 7474 4646 4848 4747 4242 4848 4343 7373 4545 4343 7373 4949 4040 5454 5050 3939 5454 4141 3939 5353 5151 CrusheenyCrusheeny 5252 BridgeBridge 5757 5555 5656 7272 5959 5858 0 50 100 6464 6161 3737 Kilometers 6666 6464 7171 8888 Client 7171 6767 6060 6565 3838 7070 6565 6262 6969 6868 6363 Project Clare River (Claregalway) Flood Relief Scheme Title Aquatic points Figure 11.411.4 Lyrr Building, IDA Business & Technology Park, Mervue, Galway, Ireland T +353 91 400200 F +353 91 400299 E [email protected] W rpsgroup.com/ireland Issue Details Drawn by: FC Project No. MGE0262 Checked by: BnC File Ref. Approved by: WM MGE0262MI0019F01 Scale: NTS Drawing No. Rev. Date: Nov 2012 MI0019 F01 Notes 1. This drawing is the property of RPS Group Ltd. It is a confidential document and must not be copied, used, or its contents divulged without prior written consent. 2. All levels are referred to Ordnance Datum, Malin Head. 3. Ordnance Survey Ireland Licence EN 0005011 ©Ordnance Survey Ireland and Government of Ireland. 3535 3131 Legend 55 ClareClare RiverRiver AquaticAquatic PointsPoints 3737 n8n8 n9n9 n8n8 n7n7 n11n11 n10n10 3838 0 50 100 Kilometers Client 3535 3131 3434 3232 Project 3636 Clare River (Claregalway) 3636 3030 Flood Relief Scheme 2929 Title 3333 3333 Aquatic points 2828 Figure 11.511.5 Lyrr Building, IDA Business & Technology Park, Mervue, Galway, Ireland T +353 91 400200 F +353 91 400299 E [email protected] W rpsgroup.com/ireland Issue Details Drawn by: FC Project No. MGE0262 Checked by: BnC File Ref. -

Appendix a Flooding and Flood Risk
Abhantrach 36 River Basin Plean um Bainistiú Priacal Tuile Flood Risk Management Plan An Éirne Erne 2018 Plean um Bainistiú Priacal Tuile Flood Risk Management Plan Amhantrach (36) An Éirne River Basin (36) Erne Limistéir um Measúnú Breise a chuimsítear sa phlean seo: Areas for Further Assessment included in this Plan: An Tulachán Tullaghan Béal an Átha Móir Ballinamore Béal Átha Beithe Ballybay Béal Átha Conaill Ballyconnell Baile an Chabháin Cavan Bun Dobhráin & máguaird Bundoran & Environs Ullmhaithe ag Oifig na nOibreacha Poiblí 2018 Prepared by the Office of Public Works 2018 De réir In accordance with Rialacháin na gComhphobal Eorpach (Measúnú agus Bainistiú Priacal Tuile) 2010 agus 2015 European Communities (Assessment and Management of Flood Risks) Regulations 2010 and 2015 Séanadh Dlíthiúil Tugadh na Pleananna um Bainistiú Priacal Tuile chun cinn mar bhonn eolais le céimeanna indéanta agus molta chun priacal tuile in Éirinn a fhreagairt agus le gníomhaíochtaí eile pleanála a bhaineann leis an rialtas. Ní ceart iad a úsáid ná brath orthu chun críche ar bith eile ná um próiseas cinnteoireachta ar bith eile. Legal Disclaimer The Flood Risk Management Plans have been developed for the purpose of informing feasible and proposed measures to address flood risk in Ireland and other government related planning activities. They should not be used or relied upon for any other purpose or decision-making process. Acknowledgements The Office of Public Works (OPW) gratefully acknowledges the assistance, input and provision of data by a large -

Irish Wildlife Manuals No. 103, the Irish Bat Monitoring Programme
N A T I O N A L P A R K S A N D W I L D L I F E S ERVICE THE IRISH BAT MONITORING PROGRAMME 2015-2017 Tina Aughney, Niamh Roche and Steve Langton I R I S H W I L D L I F E M ANUAL S 103 Front cover, small photographs from top row: Coastal heath, Howth Head, Co. Dublin, Maurice Eakin; Red Squirrel Sciurus vulgaris, Eddie Dunne, NPWS Image Library; Marsh Fritillary Euphydryas aurinia, Brian Nelson; Puffin Fratercula arctica, Mike Brown, NPWS Image Library; Long Range and Upper Lake, Killarney National Park, NPWS Image Library; Limestone pavement, Bricklieve Mountains, Co. Sligo, Andy Bleasdale; Meadow Saffron Colchicum autumnale, Lorcan Scott; Barn Owl Tyto alba, Mike Brown, NPWS Image Library; A deep water fly trap anemone Phelliactis sp., Yvonne Leahy; Violet Crystalwort Riccia huebeneriana, Robert Thompson. Main photograph: Soprano Pipistrelle Pipistrellus pygmaeus, Tina Aughney. The Irish Bat Monitoring Programme 2015-2017 Tina Aughney, Niamh Roche and Steve Langton Keywords: Bats, Monitoring, Indicators, Population trends, Survey methods. Citation: Aughney, T., Roche, N. & Langton, S. (2018) The Irish Bat Monitoring Programme 2015-2017. Irish Wildlife Manuals, No. 103. National Parks and Wildlife Service, Department of Culture Heritage and the Gaeltacht, Ireland The NPWS Project Officer for this report was: Dr Ferdia Marnell; [email protected] Irish Wildlife Manuals Series Editors: David Tierney, Brian Nelson & Áine O Connor ISSN 1393 – 6670 An tSeirbhís Páirceanna Náisiúnta agus Fiadhúlra 2018 National Parks and Wildlife Service 2018 An Roinn Cultúir, Oidhreachta agus Gaeltachta, 90 Sráid an Rí Thuaidh, Margadh na Feirme, Baile Átha Cliath 7, D07N7CV Department of Culture, Heritage and the Gaeltacht, 90 North King Street, Smithfield, Dublin 7, D07 N7CV Contents Contents ................................................................................................................................................................ -

Limerick Walking Trails
11. BALLYHOURA WAY 13. Darragh Hills & B F The Ballyhoura Way, which is a 90km way-marked trail, is part of the O’Sullivan Beara Trail. The Way stretches from C John’s Bridge in north Cork to Limerick Junction in County Tipperary, and is essentially a fairly short, easy, low-level Castlegale LOOP route. It’s a varied route which takes you through pastureland of the Golden Vale, along forest trails, driving paths Trailhead: Ballinaboola Woods Situated in the southwest region of Ireland, on the borders of counties Tipperary, Limerick and Cork, Ballyhoura and river bank, across the wooded Ballyhoura Mountains and through the Glen of Aherlow. Country is an area of undulating green pastures, woodlands, hills and mountains. The Darragh Hills, situated to the A Car Park, Ardpatrick, County southeast of Kilfinnane, offer pleasant walking through mixed broadleaf and conifer woodland with some heathland. Directions to trailhead Limerick C The Ballyhoura Way is best accessed at one of seven key trailheads, which provide information map boards and There are wonderful views of the rolling hills of the surrounding countryside with Galtymore in the distance. car parking. These are located reasonably close to other services and facilities, such as shops, accommodation, Services: Ardpatrick (4Km) D Directions to trailhead E restaurants and public transport. The trailheads are located as follows: Dist/Time: Knockduv Loop 5km/ From Kilmallock take the R512, follow past Ballingaddy Church and take the first turn to the left to the R517. Follow Trailhead 1 – John’s Bridge Ballinaboola 10km the R517 south to Kilfinnane. At the Cross Roads in Kilfinnane, turn right and continue on the R517. -

Monitoring of White-Clawed Crayfish Austropotamobius Pallipes in Irish Lakes in 2007
Monitoring of white-clawed crayfish Austropotamobius pallipes in Irish lakes in 2007 Irish Wildlife Manuals No. 37 2 Monitoring of white-clawed crayfish Austropotamobius pallipes in Irish lakes in 2007 William O’Connor 1, Gerard Hayes1, Ciaran O'Keeffe 2 & Deirdre Lynn 2 1Ecofact Environmental Consultants Ltd., Tait Business Centre, Dominic Street, Limerick City. t. +353 61 419477 f. +353 61 414315 e. [email protected] w. www.ecofact.ie 2National Parks and Wildlife Service, 7 Ely Place, Dublin 2 Citation: O’Connor, W., Hayes G., O’Keeffe, C. & Lynn, D. (2009) Monitoring of white-clawed crayfish Austropotamobius pallipes in Irish lakes in 2007. Irish Wildlife Manuals, No 37. National Parks and Wildlife Service, Department of the Environment, Heritage and Local Government, Dublin. Cover photo: Surveying for crayfish in Lough Glenade, Co. Sligo ( W. O’Connor). Irish Wildlife Manuals Series Editors: F. Marnell & N. Kingston © National Parks and Wildlife Service 2009 ISSN 1393 – 6670 SUMMARY • This report outlines the findings of a study of the Annex II listed white-clawed crayfish in 26 selected Irish lakes. The white-clawed crayfish is Ireland’s only crayfish species and Ireland is thought to hold some of the best European stocks of this species, under least threat from external factors. Lake populations of white-clawed crayfish are rare in Britain and across Europe so this adds to Ireland’s unique position in harbouring populations in lime-rich lakes. The current study sought to add to the body of existing knowledge on crayfish stocks in Irish lakes and provide a baseline reference for future studies. -

A Survey of Juvenile Lamprey Populations in the Moy Catchment
A Survey of Juvenile Lamprey Populations in the Moy Catchment Irish Wildlife Manuals No. 15 -----------------------------------------------------------------------------------------------------A Survey of Juvenile Lampreys In the Moy Catchment A Survey of Juvenile Lamprey Populations in the Moy Catchment William O’Connor Ecofact Environmental Consultants Ltd. Tait Business Centre Dominic Street Limerick City www.ecofact.ie Citation: O’Connor William (2004) A survey of juvenile lamprey populations in the Moy catchment. Irish Wildlife Manuals, No. 15. National Parks and Wildlife Service, Department of Environment, Heritage and Local Government, Dublin, Ireland. Cover photos: Images from the lamprey survey of the Moy © William O’Connor Irish Wildlife Manuals Series Editor: F. Marnell © National Parks and Wildlife Service 2004 ISSN 1393 - 6670 --------------------------------------------------------------------------------------------------------------------1---- -----------------------------------------------------------------------------------------------------A Survey of Juvenile Lampreys In the Moy Catchment TABLE OF CONTENTS EXECUTIVE SUMMARY…………………………………………………...02 1 INTRODUCTION…………………………………………………………… 04 1.1 Lampreys 2 STUDY AREA……………………………………………………………..... 10 2.1 The Moy catchment 3 METHODOLOGY…………………………………………………............... 14 3.1 Selection of sites 3.2 Electrical fishing assessment 3.3 Description of sites 3.4 Data analyses 4 RESULTS…………………………………………………...............................18 4.1 Electrical fishing sites 4.2 Site electrical -

Neagh Bann CFRAM Study
North Western - Neagh Bann CFRAM Study UoM 36 Hydrology Report IBE0700Rp0009 rpsgroup.com/ireland North Western – Neagh Bann CFRAM Study UoM 36 Hydrology Report DOCUMENT CONTROL SHEET Client OPW Project Title North Western – Neagh Bann CFRAM Study Document Title IBE0700Rp0009_UoM 36 Hydrology Report_F03 Document No. IBE0700Rp0009 DCS TOC Text List of Tables List of Figures No. of This Document Appendices Comprises 1 1 130 1 1 4 Rev. Status Author(s) Reviewed By Approved By Office of Origin Issue Date B. Quigley D01 Draft U. Mandal M. Brian G. Glasgow Belfast 08/11/2013 L. Arbuckle B. Quigley F01 Draft Final U. Mandal M. Brian G. Glasgow Belfast 31/03/2014 L. Arbuckle B. Quigley F02 Draft Final U. Mandal M. Brian G. Glasgow Belfast 11/08/2015 L. Arbuckle B. Quigley F03 Draft Final U. Mandal M. Brian G. Glasgow Belfast 08/07/2016 L. Arbuckle rpsgroup.com/ireland Copyright Copyright - Office of Public Works. All rights reserved. No part of this report may be copied or reproduced by any means without prior written permission from the Office of Public Works. Legal Disclaimer This report is subject to the limitations and warranties contained in the contract between the commissioning party (Office of Public Works) and RPS Group Ireland rpsgroup.com/ireland NW-NB CFRAM Study UoM 36 Hydrology Report – FINAL TABLE OF CONTENTS LIST OF FIGURES ................................................................................................................................. IV LIST OF TABLES ................................................................................................................................. -

County Cavan Groundwater Protection Scheme
County Cavan Groundwater Protection Scheme Volume I: Main Report Final December 2008 Jack Keyes, Sonja Masterson County Manager Groundwater Section Cavan County Council Geological Survey of Ireland Courthouse Beggars Bush Farnham Street Haddington Road, Dublin 4 Cavan Cavan Groundwater Protection Scheme. Volume I. Cavan County Council and the Geological Survey of Ireland Authors Sonja Masterson, Coran Kelly and Monica Lee, Groundwater Section, Geological Survey of Ireland with Fieldwork Assistance from: Eamon O’Loughlin, Groundwater Section, Geological Survey of Ireland and Reporting Assistance from: Caoimhe Hickey, Taly Hunter Williams, Groundwater Section, Geological Survey of Ireland in partnership with: Cavan County Council Cavan Groundwater Protection Scheme. Volume I. Cavan County Council and the Geological Survey of Ireland Cavan Groundwater Protection Scheme. Volume I. Cavan County Council and the Geological Survey of Ireland Executive Summary The Groundwater Protection Scheme for Cavan County Council provides a preliminary assessment of the relative risk to groundwater quality across the county. The main elements of the risk assessment are groundwater vulnerability (primarily subsoil thickness, subsoil permeability and karst features), aquifer potential, and source protection. The source protection element involves the delineation of protection areas around the recharge areas for selected public and group scheme groundwater supplies. The results can not be used as a substitute for site investigation for particular developments, but have proved very useful in providing County Councils with an independent, defensible, planning tool for a wide range of new developments: • Major developments (e.g. for landfill site selection, developments requiring waste management and integrated pollution licensing): helping to short-list suitable sites for detailed site investigation. -

Interim Report on the Biological Survey of River Quality
Interim Report on the Biological Survey of River Quality Results of the 2004 Investigations K.J. Clabby J. Lucey M.L. McGarrigle ENVIRONMENTAL PROTECTION AGENCY An Ghníomhaireacht um Chaomhnú Comhsaoil PO Box 3000, Johnstown Castle, Co. Wexford, Ireland Telephone: +353 5391 60600 Fax: +353 5391 60699 Email: [email protected] Website: www.epa.ie Lo Call 1890 33 55 99 © Environmental Protection Agency 2006 Although every effort has been made to ensure the accuracy of the material contained in this publication, complete accuracy cannot be guaranteed. Neither the Environmental Protection Agency nor the author accepts any responsibility whatsoever for loss or damage occasioned, or claimed to have been occasioned, in part or in full as a consequence of any person acting or refraining from acting, as a result of a matter contained in this publication. All or part of this publication may be reproduced without further permission, provided the source is acknowledged. Interim Report on the Biological Survey of River Quality Results of the 2004 Investigations Published by the Environmental Protection Agency, Ireland ISBN: 1-84095-186-9 02/06/300 Price: €15 ii PREFACE (AFF) in 1971 when some 2,900km of channel on the larger rivers and their more Section 65 (3c) of the Environmental important tributaries were biologically Protection Agency Act, 1992, requires the assessed for the first time. Since then the Environmental Protection Agency (EPA) to scope of the investigations has been “carry out, cause to be carried out, or steadily extended so that by 1990 virtually arrange for such monitoring as it may all of the rivers and streams depicted on consider necessary for the purposes of the the Ordnance Survey Map entitled "Rivers programme”. -

List of Rivers of Ireland
Sl. No River Name Length Comments 1 Abbert River 25.25 miles (40.64 km) 2 Aghinrawn Fermanagh 3 Agivey 20.5 miles (33.0 km) Londonderry 4 Aherlow River 27 miles (43 km) Tipperary 5 River Aille 18.5 miles (29.8 km) 6 Allaghaun River 13.75 miles (22.13 km) Limerick 7 River Allow 22.75 miles (36.61 km) Cork 8 Allow, 22.75 miles (36.61 km) County Cork (Blackwater) 9 Altalacky (Londonderry) 10 Annacloy (Down) 11 Annascaul (Kerry) 12 River Annalee 41.75 miles (67.19 km) 13 River Anner 23.5 miles (37.8 km) Tipperary 14 River Ara 18.25 miles (29.37 km) Tipperary 15 Argideen River 17.75 miles (28.57 km) Cork 16 Arigna River 14 miles (23 km) 17 Arney (Fermanagh) 18 Athboy River 22.5 miles (36.2 km) Meath 19 Aughavaud River, County Carlow 20 Aughrim River 5.75 miles (9.25 km) Wicklow 21 River Avoca (Ovoca) 9.5 miles (15.3 km) Wicklow 22 River Avonbeg 16.5 miles (26.6 km) Wicklow 23 River Avonmore 22.75 miles (36.61 km) Wicklow 24 Awbeg (Munster Blackwater) 31.75 miles (51.10 km) 25 Baelanabrack River 11 miles (18 km) 26 Baleally Stream, County Dublin 27 River Ballinamallard 16 miles (26 km) 28 Ballinascorney Stream, County Dublin 29 Ballinderry River 29 miles (47 km) 30 Ballinglen River, County Mayo 31 Ballintotty River, County Tipperary 32 Ballintra River 14 miles (23 km) 33 Ballisodare River 5.5 miles (8.9 km) 34 Ballyboughal River, County Dublin 35 Ballycassidy 36 Ballyfinboy River 20.75 miles (33.39 km) 37 Ballymaice Stream, County Dublin 38 Ballymeeny River, County Sligo 39 Ballynahatty 40 Ballynahinch River 18.5 miles (29.8 km) 41 Ballyogan Stream, County Dublin 42 Balsaggart Stream, County Dublin 43 Bandon 45 miles (72 km) 44 River Bann (Wexford) 26 miles (42 km) Longest river in Northern Ireland.