Language Lateralization in Monozygotic Twin Pairs
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Transfemoral Aortic Valve Implantation Using the Reverse X-Ray Image in a Patient with Dextrocardia Situs Inversus
NEW INNOVATION INTERVENTIONS FOR VALVULAR DISEASE AND HEART FAILURE Asia Intervention 2018;4: 41-44 Transfemoral aortic valve implantation using the reverse X-ray image in a patient with dextrocardia situs inversus Tomohiro Kawaguchi*, MD; Shinichi Shirai, MD; Hiroyuki Jinnouchi, MD; Kenji Ando, MD Department of Cardiology, Kokura Memorial Hospital, Kitakyushu, Japan KEYWORDS Abstract A frail 94-year-old man, who had dextrocardia situs inversus and symptomatic severe aortic stenosis, • aortic stenosis underwent transcatheter aortic valve implantation (TAVI) using the femoral approach. Computed tomo- • conduction graphy showed that there were no other cardiovascular malformations. Generally, it was difficult to main- abnormalities tain awareness of the position between his heart and ascending aorta during the procedure because of the • femoral inversion of these structures. Therefore, the reverse X-ray image was used to facilitate TAVI, and the pro- • TAVI cedure was successful without complications. However, nine days after TAVI, he developed complete atrio- ventricular block that was symptomatic. Therefore, he underwent cardiac pacemaker implantation using the reverse X-ray image to help position the atrial and ventricular leads. DOI : 10.4244/AIJ-D-17-00047 *Corresponding author: Department of Cardiology, Kokura Memorial Hospital, 3-2-1 Asano, Kokurakika, Kitakyushu, 802-0001, Japan. E-mail: [email protected] © Europa Digital & Publishing 2018. All rights reserved. SUBMITTED ON 31/10/2017 - REVISION RECEIVED ON 15/01/2018 - ACCEPTED ON 17/01/2018 41 Asia Intervention Abbreviations CT demonstrated suitable iliofemoral arteries without stenosis AS aortic stenosis or calcification that could be used as an access route. Our Heart AVB Team concluded that he was a candidate for transfemoral TAVI, atrioventricular block 2018;4: CT computed tomography because of his advanced age and frailty despite an intermediate PMI pacemaker implantation surgical risk (logistic EuroSCORE 13.6% and Society of Thoracic 41-44 SVC superior vena cava Surgeons score 7.2%). -

Isolated Levocardia with Situs Inversus Without Cardiac Abnormality in Fetus: Prenatal Diagnosis and Management
A L J O A T U N R I N R A E L P Case Report P L E R A Perinatal Journal 2020;28(1):48–51 I N N R A U T A L J O ©2020 Perinatal Medicine Foundation Isolated levocardia with situs inversus without cardiac abnormality in fetus: prenatal diagnosis and management Mucize Eriç ÖzdemirİD , Oya Demirci İD Department of Perinatology, Zeynep Kamil Maternity and Children’s Diseases Training and Research Hospital., Health Sciences University, Istanbul, Turkey Abstract Özet: Kardiyak anomalisi olmayan fetüste situs inversuslu izole levokardi: Prenatal tan› ve yönetim Objective: Isolated levocardia is a situs abnormality that the heart is Amaç: ‹zole levokardi, kalbin normal levo pozisyonunda oldu¤u in the normal levo position, but the abdominal viscera are in dextro fakat abdominal iç organlar›n dekstro pozisyonunda oldu¤u bir si- position. Most cases are accompanied by structural heart anomalies. tus anomalisidir. Ço¤u olguda yap›sal kalp anomalileri de efllik et- In this case, we aimed to present a fetus with isolated levocardia with- mektedir. Çal›flmam›zda, kardiyak anomalisi olmayan izole levo- out cardiac abnormality. kardili bir fetüsü sunmay› amaçlad›k. Case: The mother was referred to our clinic with a suspicion of fetal Olgu: Olgumuz, 22. gebelik haftas›nda fetal dekstrokardi flüphe- dextrocardia at 22 weeks of gestation. When detailed examination was siyle klini¤imize sevk edildi. Planlanan detayl› ultrason muayene- planned by ultrasonography isolated levocardia was detected in fetus. sinde, fetüste izole levokardi tespit edildi. Fetal ekokardiyografide There were no cardiac abnormalities in fetal echocardiography. Fetus hiçbir kardiyak anomali görülmedi. -

Double Outlet Right Ventricle with Dextrocardia and Situs Inversus in a Three-Year-Old Boy: a Case Report and Review of Literature
Cardiology and Angiology: An International Journal 7(3): 1-5, 2018; Article no.CA.40489 ISSN: 2347-520X, NLM ID: 101658392 Double Outlet Right Ventricle with Dextrocardia and Situs Inversus in a Three-Year-Old Boy: A Case Report and Review of Literature Rose O. Abah1,2*, Adegboyoga O. Shogo2 and Martha O. Ochoga1,2 1Department of Paediatrics, College of Health Sciences, Benue State University, Makurdi, Nigeria. 2Department of Paediatrics, Benue State University Teaching Hospital, Makurdi, Nigeria. Authors’ contributions This work was carried out in collaboration between all authors. Author ROA developed the concept, defined the intellectual content and did part of the literature search while author AOS was involved with the data acquisition and initial manuscript preparation. Author MOO contributed to the development of the intellectual concept and editing of the manuscript. All the authors reviewed and approved the final draft of the manuscript. Article Information DOI: 10.9734/CA/2018/40489 Editor(s): (1) Francesco Pelliccia, Professor, Department of Heart and Great Vessels, University La Sapienza, Rome, Italy. Reviewers: (1) Doaa Mohamed El Amrousy, Tanta University, Egypt. (2) Severin Emilia, Carol Davila University of Medicine and Pharmacy, Romania. Complete Peer review History: http://www.sciencedomain.org/review-history/23977 Received 24th January 2018 Accepted 30th March 2018 Case Report th Published 4 April 2018 ABSTRACT Aims: To highlight the rare occurrence of double outlet right ventricle (DORV) with Dextrocardia and Situs Inversus in a three-year-old boy viz-a-viz what has been reported in the literatures. Presentation of Case: N.A is a three year old boy who presented with easy fatiguebility, bluish discolouration of lips and tongue and occasional dyspnoea on exertion noticed since about 12 months of life. -

Transcatheter Device Closure of Atrial Septal Defect in Dextrocardia with Situs Inversus Totalis
Case Report Nepalese Heart Journal 2019; Vol 16(1), 51-53 Transcatheter device closure of atrial septal defect in dextrocardia with situs inversus totalis Kiran Prasad Acharya1, Chandra Mani Adhikari1, Aarjan Khanal2, Sachin Dhungel1, Amrit Bogati1, Manish Shrestha3, Deewakar Sharma1 1 Department of Cardiology, Shahid Gangalal National Heart Centre, Kathmandu, Nepal 2 Department of Internal Medicine, Kathmandu Medical College, Kathmandu,Nepal 3 Department of Pediatric Cardiology, Shahid Gangalal National Heart Centre, Kathmandu, Nepal Corresponding Author: Chandra Mani Adhikari Department of Cardiology Shahid Gangalal National Heart Centre Kathmandu, Nepal Email: [email protected] Cite this article as: Acharya K P, Adhikari C M, Khanal A, et al. Transcatheter device closure of atrial septal defect in dextrocardia with situs inversus totalis. Nepalese Heart Journal 2019; Vol 16(1), 51-53 Received date: 17th February 2019 Accepted date: 16th April 2019 Abstract Only few cases of Device closure of atrial septal defect in dextrocardia with situs inversus totalis has been reported previously. We present a case of a 36 years old male, who had secundum type of atrial septal defect in dextrocardia with situs inversus totalis. ASD device closure was successfully done. However, we encountered few technical difficulties in this case which are discussed in this case review. Keywords: atrial septal defect; dextrocardia; transcatheter device closure, DOI: https://doi.org/10.3126/njh.v16i1.23901 Introduction There are only two case reported of closure of secundum An atrial septal defect (ASD) is an opening in the atrial ASD associated in patients with dextrocardia and situs inversus septum, excluding a patent foramen ovale.1 ASD is a common totalis. -

Asplenia and Polysplenia Syndromes with Abnormalities of Lateralisation in a Sibship
J Med Genet: first published as 10.1136/jmg.18.4.301 on 1 August 1981. Downloaded from Journal of Medical Genetics, 1981, 18, 301-302 Asplenia and polysplenia syndromes with abnormalities of lateralisation in a sibship J ZLOTOGORA AND E ELIAN From the Department ofPediatrics, Hasharon Hospital, Petah-Tiqva, and Tel-Aviv University Medical School, Israel SUMMARY In the family presented here the first child had asplenia syndrome with cor biloculare' transposition of the great vessels, pulmonary stenosis, and anomalous pulmonary venous drainage' Another sib had situs inversus with polysplenia syndrome, including very similar cardiovascular defects and biliary atresia. The possibility that these two syndromes, namely asplenia and poly- splenia, are different manifestations of a similar defect in the normal asymmetrical development of internal organs is discussed. Some degree of lateral asymmetry is one of the age and weighed 2060 g. At the age of 5 days she was characteristics of the internal human body con- noted to be cyanotic and a grade 3/6 systolic murmur figuration. A mirror image of the asymmetrical was heard along the left sternal border. At cardiac organs is found in situs inversus, while in the asplenia catheterisation, transposition of the great vessels with and polysplenia syndromes there is a striking ten- double outlet of the right ventricle and pulmonary dency for internal symmetry. In the asplenia stenosis were demonstrated. The child failed to copyright. (Ivemark) syndrome the tendency is for the left lung, thrive and died at 3 months of age following an atrium, and left lobe of the liver to resemble the episode of septicaemia. -
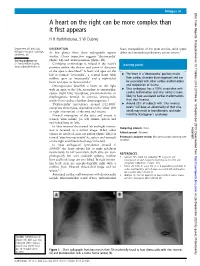
A Heart on the Right Can Be More Complex Than It First Appears
Images in... BMJ Case Reports: first published as 10.1136/bcr-2013-201046 on 19 September 2013. Downloaded from A heart on the right can be more complex than it first appears H R Haththotuwa, S W Dubrey Department of Cardiology, DESCRIPTION heart, transposition of the great arteries, atrial septal Hillingdon Hospital, Uxbridge, At first glance these chest radiographs appear defect and anomalous pulmonary venous return.3 Middlesex, UK similar. Closer inspection suggests ‘dextrocardia’ Correspondence to (figure 1A) and ‘dextroposition’ (figure 1B). ’ Dr Simon William Dubrey, Confusing terminology is helped if the heart s Learning points [email protected] position within the thorax and point of direction of the apex is described.1 A heart and apex on the ▸ ‘ ’ left is termed ‘levocardia’, a central heart with The heart in a dextrocardia position results midline apex as ‘mesocardia’ and a right-sided from cardiac chamber disarrangement and can heart and apex as ‘dextrocardia’. be associated with other cardiac malformations Dextroposition describes a heart on the right and malposition of viscera. ▸ with an apex to the left, secondary to extracardiac Situs ambiguous has a 100% association with causes (right lung hypoplasia, pneumonectomy or cardiac malformation and situs solitus is more diaphragmatic hernia). In contrast, dextrocardia likely to have associated cardiac malformations results from cardiac chamber disarrangement.2 than situs inversus. 2 ▸ ‘ ‘Dextrocardia’ (prevalence around 1/12 000) Around 20% of subjects with situs inversus ’ comprises three types, depending on the ‘situs’ (left totalis will have an abnormality of their cilia, or right orientation) of the atria and viscera. which may result in bronchiectasis and male ’ Normal orientation of the atria and viscera is infertility (Kartagener s syndrome). -

Single-Incision Laparoscopic Cholecystectomy in Situs Inversus Totalis
CASE REPORT Single-Incision Laparoscopic Cholecystectomy in Situs Inversus Totalis Mehmet Uludag, MD, Gurkan Yetkin, MD, Abdulcabbar Kartal, MD ABSTRACT INTRODUCTION Background and Objectives: Situs inversus totalis (SIT) Situs inversus totalis (SIT) is a rare congenital abnormality is a rare congenital anomaly that can cause difficulties with an autosomal recessive genetic predisposition. It during standard laparoscopic cholecystectomy due to its describes an anatomy that is a perfect mirror image of the mirror-image anatomy. These cases require more techni- normal physiologic positions of the visceral organs with cally demanding procedures, and handedness of the sur- preservation of anteroposterior relationships.1 Its inci- geon may influence performance of these operations. dence varies from one in 5000 to one in 20 000, perhaps Single-incision laparoscopic surgery (SILS) has been pro- as a reflection of very different diagnostic methods.2 posed as a less-invasive alternative to conventional lapa- In the published literature, there have been only about 40 roscopic surgery. We report the first case of successful reports in the prelaparoscopic era and about 40 reports of SILS cholecystectomy in a patient with SIT and discuss standard laparoscopic cholecystectomy in patients with technical aspects of the operation related to the handed- situs inversus. Although laparoscopic cholecystectomy ness of the surgeon. can be performed safely in patients with SIT by an expe- Case: A 49-year-old man who was known to have situs rienced laparoscopic surgeon, laparoscopic cholecystec- inversus totalis presented with symptomatic cholelithiasis. tomy in SIT is technically more demanding than in pa- This patient was operated on by a right-handed surgeon. -

ISPUB.COM Volume 11 Number 1
The Internet Journal of Cardiology ISPUB.COM Volume 11 Number 1 Dextrocardia, Situs Inversus And Multiple Congenital Cardiac Defects In A Nigerian Infant C Duru, B Otaigbe Citation C Duru, B Otaigbe. Dextrocardia, Situs Inversus And Multiple Congenital Cardiac Defects In A Nigerian Infant. The Internet Journal of Cardiology. 2013 Volume 11 Number 1. Abstract Situs inversus with dextrocardia is the complete reversal of the position of the abdominal and thoracic viscera. It may remain asymptomatic or could be detected early in infancy when associated with other congenital malformations especially of the cardiovascular system. Situs inversus with dextrocardia usually exists without co-existing congenital heart disease. We report the case of a seven month old Nigerian male infant who presented with complaints of recurrent cough and bluish discoloration of the lips. Examination revealed central cyanosis with grade 3 digital clubbing and a right-sided apical impulse. Chest radiograph showed a right sided heart and Echocardiogram confirmed multiple cardiac anomalies (Complete Atrio-ventricular septal defect (AVSD), Pulmonary atresia and Patent ductus arteriosus. INTRODUCTION and there was no murmur. There was no evidence of cardiac Dextrocardia is a congenital anomaly in which the heart is failure. situated on the right side of the body1. Situs inversus is a He was the second of two children and the product of term condition in which the major visceral organs are mirrored gestation delivered by spontaneous vertex to a 32 year old from their normal positions, that is the morphological left mother in a non-consanguineous marriage. There was no atrium is on the right while the morphological right atrium, history of exanthematous febrile illness, ingestion of herbal the liver and gall bladder are on the left.2 This is otherwise concoctions or exposure to irradiation in pregnancy. -

Situs Inversus- Not Just a Mirror Image
Jebmh.com Case Report Situs Inversus- Not Just a Mirror Image Bhavya Krishna1, Nidhi Pathak2 1Senior Resident, Department of Anaesthesia, VMMC and Safdarjung Hospital, New Delhi, India. 2Senior Resident, Department of Anaesthesia, VMMC and Safdarjung Hospital, New Delhi, India. INTRODUCTION Situs inversus totalis, a rare condition presents with multitude of problems due to Corresponding Author: Dr. Nidhi Pathak, multiple structural and functional abnormalities that coexist with it like cardiac, #B-7, B- Block, Main Road, spine and airway malformations. We are reporting a patient who had situs inversus New Ashok Nagar, New Delhi- 110096, totalis and was operated for upper limb fracture. The present case report India. emphasises on the challenges during anaesthetic management of a patient with E-mail: [email protected] situs inversus. Situs inversus (also called situs transversus or oppositus) is a congenital DOI: 10.18410/jebmh/2020/182 condition in which the major thoracic and abdominal visceral organs are reversed Financial or Other Competing Interests: 1 or mirrored from their normal positions. It is a rare disease with a predicted None. incidence of one in 10,000 among the general population.2 We are reporting a case of situs inversus totalis who was operated for upper limb fracture for which How to Cite This Article: we gave a nerve block, an anaesthesia modality which has not been published in Bhavya K, Pathak N. Situs inversus- not just a mirror image. J. Evid. Based Med. a situs inversus patient earlier. These patients pose a challenge to Healthc. 2020; 7(16), 840-842. DOI: anaesthesiologists because of the associated conditions like Kartagener's 10.18410/jebmh/2020/182 syndrome, cardiac anomalies, spleen malformations and other such clinical entities, all of which have specific anaesthetic implications, and not just for the Submission 17-03-2020, Peer Review 23-03-2020, anatomical mirroring. -

Ischemic Mitral Regurgitation in a Patient with Dextrocardia and Situs Inversus Totalis Husain Esmaeil1, Jamal Al-Fadhli1, Abdullah Dashti2, and Nael Al-Sarraf1,*
Journal of Surgical Case Reports, 2019;11, 1–3 doi: 10.1093/jscr/rjz329 Case Report CASE REPORT Ischemic mitral regurgitation in a patient with dextrocardia and situs inversus totalis Husain Esmaeil1, Jamal Al-Fadhli1, Abdullah Dashti2, and Nael Al-Sarraf1,* 1Department of Cardiac Surgery, Chest Diseases Hospital, Kuwait, and 2Department of Cardiac Anesthesia, Chest Diseases Hospital, Kuwait. *Correspondence address. Department of Cardiac surgery, Chest Diseases Hospital, Al-Jabriah, PO Box 1134, Postal Code 46312, Kuwait. Tel: +965 98882921; Fax: +96265859378; E-mail: [email protected] Abstract Dextrocardia with situs inversus is a rare condition. Few previously published cases have addressed the observation of isolated coronary artery disease or isolated mitral valve disease in these patients. However, the occurrence of ischemic mitral regurgitation in such cases has never been previously reported. Here, we report such a case with emphasis on both surgical and echocardiographic features. INTRODUCTION severe left ventricular dysfunction (ejection fraction 30%), severe Ischemic mitral regurgitation is rare in patients with dextrocar- global left ventricular hypokinesia and severe ischemic mitral dia and situs inversus (SI) totalis. Careful planning and adequate regurgitation with eccentric jet directed anteriorly and tethered surgical set-up are important when dealing with such case. posterior mitral valve leaflet. He was stabilized medically and Transesophageal echocardiography (TEE) should be interpreted was referred for surgery. carefully using modification from the standard views to aid in He underwent Coronary Artery Bypass Graft (CABG) and understanding mitral valve. mitral valve repair through a median sternotomy utilizing cardiopulmonary bypass (CPB) and cardioplegia cardiac arrest. Surgeon was standing on the left side of the patient. -

Double Valve Replacement in a Patient with Dextrocardia and Situs Inversus Toatalis: a Case Report Mohammed Firoj Khan1 and Wen Zeng Zhao2
Case Report iMedPub Journals Annals of Clinical and Laboratory Research 2016 http://www.imedpub.com/ Vol.4 No.2:102 ISSN 2386-5180 Double Valve Replacement in a Patient with Dextrocardia and Situs Inversus Toatalis: A Case Report Mohammed Firoj Khan1 and Wen Zeng Zhao2 1Department of Cardio-vascular Surgery, 2nd Affiliated Hospital of Zhengzhou University, Zhengzhou, Henan, PR China 2Department of Cardio-vascular Surgery, 1st Affiliated Hospital of Zhengzhou University, Zhengzhou, Henan, PR China Corresponding author: Wen Zeng Zhao, Department of Cardio-vascular Surgery, 1st affiliated hospital of Zhengzhou University, Zhengzhou, Henan, PR China, Tel: 008613603996718; E-mail: [email protected] Received: 31 May 2016; Accepted: 18 June 2016; Published: 22 June 2016 Citation: Khan MF, Zhao WZ. Double Valve Replacement in a Patient with Dextrocardia and Situs Inversus Toatalis: A Case Report. Ann Clin Lab Res. 2016, 4: 2. in different studies [4,5]. Dextrocardia with situs inversus totalis means, heart and all other body organs are the mirror Abstract image of one another [6]. The incidence of dextrocardia with situs inversus totalis is 1/10,000 to 50,000 births [7]. Dextrocardia is a rare congenital cardiac anomaly with the Dextrocardia in adults are usually diagnosed incidentally heart in the right side of the chest which can often be during routine examination or radiographic examination for associated with other complex congenital heart diseases some purposes. Dextrocardia is also confused with such as a single ventricle, double outlet or double inlet dextroposition in which the heart is displaced towards the ventricles and tricuspid atresia. right hemithorax due to some abnormalities such as diaphragmatic hernia, right pneumonectomy, or right A 51-years- old man diagnosed with dextrocardia in a local pulmonary hypoplasia etc [8]. -

Heart on the Right May Sometimes Be ‘Right’
THE CLINICAL PICTURE PRASHANT SHARMA, MD VIJAIGANESH NAGARAJAN, MD, MRCP DONALD A. UNDERWOOD, MD Department of Hospital Internal Medicine, Department of Cardiovascular Medicine, University of Department of Cardiovascular Medicine, Mayo Clinic, Rochester, MN Virginia, Charlottesville Cleveland Clinic Heart on the right may sometimes be ‘right’ Negative complexes FIGURE 1. The standard left-sided 12-lead electrocardiogram showed right-axis deviation; inverted P, QRS, and T waves in leads I and aVL (arrows), and positive complexes in lead in lead I, aVR (circle). Leads V1–V6 showed reversal of R-wave progression. positive complexes 76-year-old man presented to the In patients with dextrocardia, right-sid- A emergency department with right-sided ed hookup of the electrodes is usually neces- in aVR, and exertional chest pain radiating to the right sary for proper interpretation of the ECG. slight reversal shoulder and arm associated with shortness of When this was done in our patient, the breath. His vital signs were normal. On clini- ECG showed a normal cardiac axis, a nega- of R-wave cal examination, the cardiac apex was palpat- tive QRS complex in lead aVR, a positive progression ed on the right side, 9 cm from the midsternal P wave and other complexes in lead I, and line in the fi fth intercostal space. normal R-wave progression in the precordial indicate A standard left-sided 12-lead electrocar- leads—fi ndings suggestive of dextrocardia dextrocardia diogram (ECG) showed right-axis deviation (FIGURE 2). and inverted P, QRS, and T waves in leads I Chest radiography showed a right-sided and aVL (FIGURE 1).