Frontal Lobes and Neuropsychiatric Illness This Page Intentionally Left Blank the Frontal Lobes and Neuropsychiatric Illness
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Traumatic Brain Injury
REPORT TO CONGRESS Traumatic Brain Injury In the United States: Epidemiology and Rehabilitation Submitted by the Centers for Disease Control and Prevention National Center for Injury Prevention and Control Division of Unintentional Injury Prevention The Report to Congress on Traumatic Brain Injury in the United States: Epidemiology and Rehabilitation is a publication of the Centers for Disease Control and Prevention (CDC), in collaboration with the National Institutes of Health (NIH). Centers for Disease Control and Prevention National Center for Injury Prevention and Control Thomas R. Frieden, MD, MPH Director, Centers for Disease Control and Prevention Debra Houry, MD, MPH Director, National Center for Injury Prevention and Control Grant Baldwin, PhD, MPH Director, Division of Unintentional Injury Prevention The inclusion of individuals, programs, or organizations in this report does not constitute endorsement by the Federal government of the United States or the Department of Health and Human Services (DHHS). Suggested Citation: Centers for Disease Control and Prevention. (2015). Report to Congress on Traumatic Brain Injury in the United States: Epidemiology and Rehabilitation. National Center for Injury Prevention and Control; Division of Unintentional Injury Prevention. Atlanta, GA. Executive Summary . 1 Introduction. 2 Classification . 2 Public Health Impact . 2 TBI Health Effects . 3 Effectiveness of TBI Outcome Measures . 3 Contents Factors Influencing Outcomes . 4 Effectiveness of TBI Rehabilitation . 4 Cognitive Rehabilitation . 5 Physical Rehabilitation . 5 Recommendations . 6 Conclusion . 9 Background . 11 Introduction . 12 Purpose . 12 Method . 13 Section I: Epidemiology and Consequences of TBI in the United States . 15 Definition of TBI . 15 Characteristics of TBI . 16 Injury Severity Classification of TBI . 17 Health and Other Effects of TBI . -

Emotional Disorders in Patients with Cerebellar Damage - Case Studies
Should be cited as: Psychiatr. Pol. 2014; 48(2): 289–297 PL ISSN 0033-2674 www.psychiatriapolska.pl Emotional disorders in patients with cerebellar damage - case studies Katarzyna Siuda 1, Adrian Andrzej Chrobak 1, Anna Starowicz-Filip2, Anna Tereszko1, Dominika Dudek 3 1Students’ Scientific Association of Adult Psychiatry, Jagiellonian University Medical College, Tutor: prof. dr hab. med. D. Dudek 2Medical Psychology Department, Jagiellonian University Medical College, Head: prof. dr hab. n. hum. J.K. Gierowski 3Department of Affective Disorders, Jagiellonian University Medical College Head: prof. dr hab. med. D. Dudek Summary Aim. Growing number of research shows the role of the cerebellum in the regulation of affect. Lesions of the cerebellum can lead to emotional disregulation, a significant part of the Cerebellar Cognitive Affective Syndrome. The aim of this article is to analyze the most recent studies concerning the cerebellar participation in emotional reactions and to present three cases: two female and one male who suffered from cerebellar damage and presented post-traumatic affective and personality change. Method. The patients’ neuropsychological examination was performed with Raven’s Progressive Matrices Test – standard version, Trial Making Test, Wisconsin Card Sorting Test, Auditory Verbal Learning Test by Łuria, Benton Visual Retention Test, Verbal Fluency Test, Stroop Interference Test, Attention and Perceptivity Test (Test Uwagi i Spostrzegawczości TUS), Frontal Behavioral Inventory (FBI). Results. The review of the literature suggest cerebellar participation, especially the vermis and paravermial regions, in the detection, integration and filtration of emotional information and in regulation of autonomic emotional responses. In the described patients we observed: oversensitivity, irritability, impulsivity and self-neglect. -

Frontal Stroke: Problem Solving, Decision Making, Impulsiveness
PSYCHOLOGY Psychology & Neuroscience, 2011, 4, 2, 267 - 278 NEUROSCIENCE DOI: 10.3922/j.psns.2011.2.012 Frontal stroke: problem solving, decision making, impulsiveness, and depressive symptoms in men and women Morgana Scheffer1, Janine Kieling Monteiro1 and Rosa Maria Martins de Almeida2 1 - Universidade do Vale do Rio dos Sinos, RS, Brazil 2 - Universidade Federal do Rio Grande do Sul, Porto Alegre, RS, Brazil Abstract The present study compared men and women who suffered a frontal lobe stroke with regard to problem solving, decision making, impulsive behavior and depressive symptoms and also correlated these variables between groups. The sample was composed of 10 males and nine females. The study period was 6 months after the stroke. The following instruments were used: Wisconsin Card Sort Test (WCST), Iowa Gambling Task (IGT), Barrat Impulsiveness Scale (BIS11), and Beck Depression Inventory (BDI). For the exclusion criteria of the sample, the Mini International Psychiatric Interview (M.I.N.I Plus) and Mini Mental Stage Examination (MMSE) were used. To measure functional severity post-stroke, the Rankin Scale was used. The average age was 60.90 ± 8.93 years for males and 60.44 ± 11.57 years for females. In females, total impulsiveness (p = .013) and lack of planning caused by impulsiveness (p = .028) were significantly higher compared with males, assessed by the BIS11. These data indicate that females in the present sample who suffered a chronic frontal lesion were more impulsive and presented more planning difficulties in situations without demanding cognitive processing. These results that show gender differences should be considered when planning psychotherapy and cognitive rehabilitation for patients who present these characteristics. -

Reading Braniacs
Reading Brainiacs Adeetee Bhide, University of Pittsburgh Unlike learning to speak, learning to read and write requires years of explicit instruction. However, once a person learns to read well, reading is effortless, and in fact, obligatory. Skilled adult readers cannot refrain from reading, even if reading is impairing their performance. To demonstrate this, take the very simple Stroop test 1 In the lists below, try to name the ink color of each word out loud (so, for the first list you would say "red, green, blue…”. List 1 List 2 Table Computer Green Purple Chair Sofa Red Red Book Plate Green Green Paper Mug Blue Purple Which list did you find easier? The first list was probably much easier than the second. That is because, even though the written words are completely irrelevant to the task, as skilled adult readers we cannot stop reading them. In the first list, the written words are simply random objects. However, in the second list, the written words are the incorrect colors, and they interfere with our production of the correct answer. How do we go from struggling to read, sounding out one letter at a time, to becoming such skilled readers that we cannot even prevent ourselves from reading? And how does the brain change during the process of becoming a skilled reader? This article examines the neural mechanisms that underlie our ability to read. The beginning largely focuses on English, because the majority of scientific research on reading has been done with English. Later, the article covers the neural mechanisms behind reading other languages, including the akshara languages. -

Nonverbal Processing in Frontotemporal Lobar Degeneration
Nonverbal processing in frontotemporal lobar degeneration Dr Rohani Omar MA MRCP Submitted to University College London for the degree of MD(Res) 1 SIGNED DECLARATION I, Rohani Omar confirm that the work presented in this thesis is my own. Where information has been derived from other sources, I confirm that this has been indicated in the thesis. 2 ABSTRACT Frontotemporal lobar degeneration (FTLD) refers to a group of diseases characterised by focal frontal and temporal lobe atrophy that collectively constitute a substantial source of clinical and social disability. Patients exhibit clinical syndromes that are dominated by a variety of nonverbal cognitive and behavioural features such as agnosias, altered emotional and social responses, impaired regulation of physiological drives, altered chemical sense, somatosensory and interoceptive processing. Brain mechanisms for processing nonverbal information are currently attracting much interest in the basic neurosciences and deficits of nonverbal processing are a major cause of clinical symptoms and disability in FTLD, yet these clinical deficits remain poorly understood and accurate diagnosis is often difficult to achieve. Moreover, the cognitive and neuroanatomical correlates of behavioural and nonverbal cognitive syndromes in FTLD remain largely undefined. The experiments described in this thesis aim to address the issues of improving our understanding of the social and behavioural symptoms in FTLD through the integration of detailed neuro-behavioural, neuropsychological and neuroanatomical analyses of a range of nonverbal functions (including emotions, sounds, odours and flavours) with high- resolution structural magnetic resonance imaging (MRI). A prospective study of emotion recognition in various domains including music, faces and voices shows that music is especially vulnerable to the effects of damage in FTLD. -

Frontal Lobe Syndrome Treatment Pdf
Frontal lobe syndrome treatment pdf Continue Mesulam MM. Human frontal lobes: Overcoming the default mode through the coding of the continent. DT Stus and RT Knight. Principles of frontal lobe function. Oxford: 2002. 8-30. Bonelli RM, Cummings JL. Frontal subcortial circuit and behavior. Dialogues Wedge Neuroshi. 2007. 9(2):141-51. (Medline). Cruz-Oliver DM, Malmstrom TK, Allen CM, Tumosa N, Morley JE. University of St. Louis Veterans' Examination for Mental Status (SLUMS Exam) and Mini-Mental Status Examination as Predictors of Mortality and Institutionalization. J Natra is a health aging. 2012. 16(7):636-41. (Medline). Dubois B, Slachevsky A, Litvin I, Pillon B. FAB: Front battery scores at the bedside. Neurology. 2000 Dec 12. 55(11):1621-6. (Medline). Nasreddin S.S., Phillips N.A., Sdirian V et al. Montreal Cognitive Assessment, MoCA: A Short Screening Tool for Mild Cognitive Impairment. J Am Geriatr Soc. 2005 Apr 53 (4):695-9. (Medline). Copp B, Russer N, Tabeling S, Steurenburg Headquarters, De Haan B., KarnatHO, etc. Performance on the front battery scores are sensitive to frontal lobe damage in stroke patients. BMC Neurol. 2013 November 16. 1:179 p.m. (Medline). (Full text). Munoz DP, Everling S. Otadi: anti-sacdad task and voluntary eye control. Nat Rev Neuroshi. March 5, 2004. 5:218-28. (Medline). (Full text). Reitan RM. Trace ratio by doing a test for organic brain damage. J Consult Psychol. 1955 October 19 (5):393-4. (Medline). Mall J, de Oliveira-Souza R, Mall FT, Bramati IE, Andreiuolo PA. Cerebral correlates with a change in the set: an MRI track study while doing a test. -

TESIS DOCTORAL Apnea Obstructiva Del Sueño Y Funcionamiento
DEPARTAMENTO DE PSICOLOGÍA BÁSICA, PSICOBIOLOGIA Y METODOLOGÍA DE LAS CIENCIAS DEL COMPORTAMIENTO TESIS DOCTORAL Apnea Obstructiva del Sueño y Funcionamiento Ejecutivo Paulo Jorge Sargento dos Santos Salamanca 2015 Dª. Mª VICTORIA PEREA BARTOLOME. Dra. en Medicina y Cirugía. Especialista en Neurología. Catedrática de Universidad. Area de Psicobiología. Dpto. de Psicología Básica, Psicobiología y Metodología de las Ciencias del Comportamiento. Facultad de Psicología. Universidad de Salamanca. Dª. VALENTINA LADERA FERNANDEZ. Dra. en Psicología. Profesora Titular de Universidad. Area de Psicobiología. Dpto. de Psicología Básica, Psicobiología y Metodología de las Ciencias del Comportamiento. Facultad de Psicología. Universidad de Salamanca. CERTIFICAN: Que el trabajo, realizado bajo nuestra dirección por D. PAULO JORGE SARGENTO DOS SANTOS, licenciado en Psicología y alumno del Programa de Doctorado “Neuropsicología Clínica” titulado, “Apnea Obstructiva del Sueño y Funcionamiento Ejecutivo”, reúne los requisitos necesarios para optar al GRADO DE DOCTOR por la Universidad de Salamanca. Salamanca, abril de 2015 Fdo.: Mª Victoria Perea Bartolomé Fdo.: Valentina Ladera Fernández El dulce recuerdo de mis abuelos, José y João, mis abuelas, Ilda y Aida, mi tío Carlos, mis amigos Xico e Rui Pimpão Agradecimientos Agradecimientos y reconocimientos Lo primero y más importante de todo, quiero agradecer a las Directoras de esta tesis, Profesora Dra. María Victoria Perea y Profesora Dra. Valentina Ladera, que la han dirigido mediante la promoción de la autonomía y la búsqueda del conocimiento, sino también, como se ya se ha indicado en otra parte, me han llevado por el feliz hallazgo de la Neuropsicología: ¡MUCHAS GRACIAS, MAESTRAS! A la Universidad de Salamanca, por darme el gran privilegio de ser su alumno. -

HMSA Payment Transformation 2020 Measure Value Set Breast Cancer
PT - 2020 Measure Value Set_Breast Cancer Screening Numerator Value Set Name Code Definition Code System Mammography 77055 CPT Mammography 77056 CPT Mammography 77057 CPT Mammography 77061 CPT Mammography 77062 CPT Mammography 77063 CPT Mammography 77065 CPT Mammography 77066 CPT Mammography 77067 CPT Screening mammography, bilateral (2-view study of each breast), including computer-aided Mammography G0202 HCPCS detection (cad) when performed (G0202) Diagnostic mammography, including computer-aided detection (cad) when performed; Mammography G0204 HCPCS bilateral (G0204) Diagnostic mammography, including computer-aided detection (cad) when performed; Mammography G0206 HCPCS unilateral (G0206) Mammography 87.36 Xerography of breast ICD9PCS Mammography 87.37 Other mammography ICD9PCS Mammography 24604-1 MG Breast Diagnostic Limited Views LOINC Mammography 24605-8 MG Breast Diagnostic LOINC Mammography 24606-6 MG Breast Screening LOINC Mammography 24610-8 MG Breast Limited Views LOINC Mammography 26175-0 MG Breast - bilateral Screening LOINC Mammography 26176-8 MG Breast - left Screening LOINC Mammography 26177-6 MG Breast - right Screening LOINC Mammography 26287-3 MG Breast - bilateral Limited Views LOINC Mammography 26289-9 MG Breast - left Limited Views LOINC Mammography 26291-5 MG Breast - right Limited Views LOINC Mammography 26346-7 MG Breast - bilateral Diagnostic LOINC Mammography 26347-5 MG Breast - left Diagnostic LOINC Mammography 26348-3 MG Breast - right Diagnostic LOINC Mammography 26349-1 MG Breast - bilateral Diagnostic -

Brain Injury and Theschools
BRAIN INJURY AND THE SCHOOLS A GUIDE FOR EDUCATORS Brain Injury Association of Virginia 804-355-5748 800-444-6443 www.biav.net Table of Contents Acknowledgements Foreword A. Brain Injury 101 This Is Your Brain A1 The Brain: What Side Are You On? A2 An Introduction to Brain Injury A3 Traumatic Brain Injury vs. Non-Traumatic Brain Injury A7 Potential Damage in a Closed Brain Injury A8 Potential Damage in an Open Brain Injury A9 Major Causes of Brain Injury in Children A10 Sports Injury and Concussion A11 B. The Student With TBI: An Overview Introduction B1 Determining Present Level of Educational Performance B2 Simple Math B3 Subject Area Challenges and Solutions (Math, Science, Reading, Writing) B4 Distinguishing Traumatic Brain Injury (TBI) from Other Disabilities B8 Neuropsychological Testing B15 Standardized Evaluations Appropriate for Children with TBI B17 C. Educational Implications Introduction C1 Cognitive C2 Behavioral C8 Motor-Sensory C24 Accommodation Strategies C29 D. Transition Introduction D1 General Principles of Transition D2 Transition Planning Worksheet D5 Individualized Health Care Plan D8 Transition Strategies D11 Transition Resources D13 E. Family What are the Families Going Through? E1 What Support Can be Provided to Families? E3 2013 Brain Injury Association of Virginia 1506 Willow Lawn Dr., Suite 212 Richmond, VA 23230 804-355-5748 www.biav.net F. Special Education Introduction F1 IDEA (Individuals with Disabilities Act) and 504 F2 A Very Brief Introduction F3 The Individualized Education Plan (IEP) and the IEP Team F4 Services F5 Parental Rights and Procedural Safeguards F7 Resolving Disagreements F8 What’s In an IEP? F9 IEP Teams F10 Criteria for Developing Appropriate IEP Goals F11 G. -

Frontal Lobe Traumatic Brain Injuries and Executive Dysfunctioning Sydney L
Southern Illinois University Carbondale OpenSIUC Research Papers Graduate School 2013 Frontal Lobe Traumatic Brain Injuries and Executive Dysfunctioning Sydney L. Goss Southern Illinois University Carbondale, [email protected] Follow this and additional works at: http://opensiuc.lib.siu.edu/gs_rp Recommended Citation Goss, Sydney L., "Frontal Lobe Traumatic Brain Injuries and Executive Dysfunctioning" (2013). Research Papers. Paper 348. http://opensiuc.lib.siu.edu/gs_rp/348 This Article is brought to you for free and open access by the Graduate School at OpenSIUC. It has been accepted for inclusion in Research Papers by an authorized administrator of OpenSIUC. For more information, please contact [email protected]. FRONTAL LOBE TRAUMATIC BRAIN INJURIES AND EXECUTIVE DYSFUNCTIONING by Sydney Goss B.S., University of Wisconsin - Madison, 2011 A Research Paper Submitted in Partial Fulfillment of the Requirements for the Master of Art Degree. Rehabilitation Institute in the Graduate School Southern Illinois University Carbondale May 2013 RESEARCH PAPER APPROVAL FRONTAL LOBE TRAUMATIC BRAIN INJURY AND EXECUTIVE DYSFUNCTION By Sydney Goss A Research Paper Submitted in Partial Fulfillment of the Requirements for the Degree of Master of Arts in the field of Communication Disorders and Sciences Approved by: Dr. Kenneth O. Simpson, Chair Dr. Maria Claudia Franca, Ph.D., CCC-SLP Kathryn Martin, M.S., CCC-SLP Graduate School Southern Illinois University Carbondale March 25, 2013 TABLE OF CONTENTS PAGE INTRODUCTION……………………………………………………………………….1 -

Brain Injury Psychopharmacology: Understanding Symptoms, Syndromes and Medication Interventions A.J
Brain Injury Psychopharmacology: Understanding Symptoms, Syndromes and Medication Interventions A.J. Zolten, Ph.D., Director of Neuropsychology and Psychology Services, NeuroRestorative TimberRidge Learning Objectives • Participants will learn about brain injury dynamics and neuroanatomy of brain injury. • Participants will learn about syndromes specific to brain anatomy. • Participants will learn about medication interventions for differing syndromes related to brain injury deficits and dysfunction throughout the recovery process. Disclosures and Disclaimers • I am not a physician and I do not prescribe medications. I am a neuropsychologist with 23 years experience in brain injury rehabilitation and treatment and will be describing treatments based upon experience and education in Brain Injury Rehabilitation. • Many medications described in this presentation will be “off label” with regard to FDA approval for primary use. I do not personally or professionally recommend that anyone who is present today (or reads this presentation at some other time) make decisions regarding prescription of ANY medications based solely upon this presentation. • Always use good clinical judgment, the counsel of educated and well trained professionals, and published information when deciding on use of medications. When the Force is Not with You: Dynamics of a Brain Injury Trauma to the head generates many injuries • Direct Impact to the Skull • Coup-Contracoup Injuries • Shear and Strain Injuries • Brain-Blood vessel Injuries • Swelling of Bruised Tissue Coup-Contra Coup Damage • Damage Occurs in a direct line from • When forces rotate, extra force impact through the brain stretches and shears the underlying white matter Skull and White Matter: Brain Support that Matters • Base of the Skull is Bony and Rough • White matter consists of bundles of axonal “wires” that transmit Neuron signals to other • Brain Physically moves on top of the parts of the Cortex. -
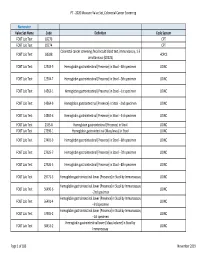
2020 Measure Value Set Colorectal Cancer Screening
PT ‐ 2020 Measure Value Set_Colorectal Cancer Screening Numerator Value Set Name Code Definition Code System FOBT Lab Test 82270 CPT FOBT Lab Test 82274 CPT Colorectal cancer screening; fecal occult blood test, immunoassay, 1‐3 FOBT Lab Test G0328 HCPCS simultaneous (G0328) FOBT Lab Test 12503‐9 Hemoglobin.gastrointestinal [Presence] in Stool ‐‐4th specimen LOINC FOBT Lab Test 12504‐7 Hemoglobin.gastrointestinal [Presence] in Stool ‐‐5th specimen LOINC FOBT Lab Test 14563‐1 Hemoglobin.gastrointestinal [Presence] in Stool ‐‐1st specimen LOINC FOBT Lab Test 14564‐9 Hemoglobin.gastrointestinal [Presence] in Stool ‐‐2nd specimen LOINC FOBT Lab Test 14565‐6 Hemoglobin.gastrointestinal [Presence] in Stool ‐‐3rd specimen LOINC FOBT Lab Test 2335‐8 Hemoglobin.gastrointestinal [Presence] in Stool LOINC FOBT Lab Test 27396‐1 Hemoglobin.gastrointestinal [Mass/mass] in Stool LOINC FOBT Lab Test 27401‐9 Hemoglobin.gastrointestinal [Presence] in Stool ‐‐6th specimen LOINC FOBT Lab Test 27925‐7 Hemoglobin.gastrointestinal [Presence] in Stool ‐‐7th specimen LOINC FOBT Lab Test 27926‐5 Hemoglobin.gastrointestinal [Presence] in Stool ‐‐8th specimen LOINC FOBT Lab Test 29771‐3 Hemoglobin.gastrointestinal.lower [Presence] in Stool by Immunoassay LOINC Hemoglobin.gastrointestinal.lower [Presence] in Stool by Immunoassay FOBT Lab Test 56490‐6 LOINC ‐‐2nd specimen Hemoglobin.gastrointestinal.lower [Presence] in Stool by Immunoassay FOBT Lab Test 56491‐4 LOINC ‐‐3rd specimen Hemoglobin.gastrointestinal.lower [Presence] in Stool by Immunoassay FOBT Lab Test 57905‐2