Elia™ Dsdna – the Answer in SLE Testing
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Immunocap Specific
® Reagents for Phadia 250 • Development Solution (Art No 10-9314-01: for 6 x 2000 determinations) ImmunoCAP Specific IgE • ImmunoCAP Specific IgE Conjugate 100 (Art No 10-9316-01: for 6 x 100 • Stop Solution (Art No 34-2337-11: for 4600 determinations) determinations) • Washing Solution Additive (Art No 10-9518-01: 4 x 850 ml) Fluoroenzymeimmunoassay • Washing Solution Concentrate (Art No 34-2337-21: 1 x 2800 ml) Calibrator Range 0-100 kU/l • ImmunoCAP Specific IgE Conjugate 400 (Art No 10-9310-01: for 6 x 400 determinations) • ImmunoCAP Specific IgE Control (Art No 10-9449-01: for 6 x 4 determinations) Directions for Use 52-5291-EN/05 • ImmunoCAP Specific IgE Calibrator Strip 0-100 (Art No 10-9459-01: for 5 calibration • ImmunoCAP Specific IgE f1 Control (Art No 10-9450-01: for 4 x 4 determinations) curves) • ImmunoCAP Specific IgE Control L (Art No 10-9528-01: for 6 x 4 determinations) INTENDED USE • ImmunoCAP Specific IgE Curve Control Strip (CC-1 and CC-2) (Art No 10-9312-01: • ImmunoCAP Specific IgE Control M (Art No 10-9529-01: for 6 x 4 determinations) ImmunoCAP Specific IgE is an in vitro test system for the quantitative measurement of 5 x 3 sets of curve control) • ImmunoCAP Specific IgE Control H (Art No 10-9530-01: for 6 x 4 determinations) allergen specific IgE in human serum or plasma. It is intended for in vitro diagnostic use as • ImmunoCAP Specific IgE Anti-IgE (a_IgE) (Art No 14-4417-01: carriers of 16 • ImmunoCAP Specific IgE Negative Control (Art No 10-9445-01: for 6 x 4 an aid in the clinical diagnosis of IgE mediated allergic disorders in conjunction with other ImmunoCAP) determinations) clinical findings, and is to be used in clinical laboratories. -
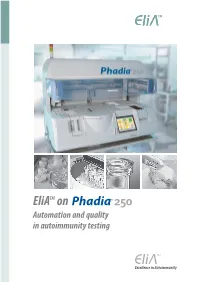
Elia™ on Phadia® 250 – the Future in Autoimmunity Testing –Now!
EliATM on Automation and quality in autoimmunity testing EliA™ on Phadia® 250 – the Future in Autoimmunity Testing –Now! High Throughput n 350 or more tests per day n additional throughput with over-night runs n results in one-minute intervals n continuous access for samples Highly Automated n true walk-away n automatic „wake-up“ and shut down n barcode reading for all reagents and samples to minimise errors n remote monitoring for easy troubleshooting n primary and secondary tubes accepted Flexibility n patient-specific test profiles n autoimmunity and allergy in one run n small runs cost-efficient n priority function for urgent samples n reflex testing Quality n accurate, standardised and reproducible results n custom-designed, reliable technology dedicated to antibody testing n reagents produced under GMP conditions n regular, system-specific quality control with Quality Club™ EliA™ Autoimmunity The EliA™ System n Time for the Phadia® 250 is a highly automated system for autoimmuni ty essentials (EliA™) and allergy (ImmunoCAP™) testing. Designed to assist laboratory workflow by reducing hands-on time, it processes samples from test request to final result with minimal inter- vention. Barcode identification of all reagents, mainframe worklist downloads or barcode/manual sample identification combine with on-board dilution to reduce manual handling and minimise errors. Over-night runs with automatic shut down after the last sample and the automated „wake-up“ give the option of in creasing the available working hours of the instrument. All reagents can be stored on board to minimise loading times. User-friendly operation makes test handling and data proces- sing simple and convenient. -

Immunodiagnostics | Journal No
Journal No. 7. 2013 Scientific news, opinions and reports ImmunoJournalDiagnostics 8th EliA Symposium: Autoimmune Gastrointestinal Diseases Our 8th EliA Symposium in Freiburg took place from May 26th - 27th, 2013. More than 220 guests came to this symposium where eight excellent speakers held presentations on inflammatory bowel diseases and celiac disease, their clinical presentations, therapy and diagnostic tools. Additionally, we invited scientists from Europe to present their studies and received 15 posters on very diverse topics such as the comparison of different calprotectin stool tests, cost effectiveness of calprotectin or HLA testing in celiac disease. These posters are reprinted in this issue of the Immuno Diagnostics Journal. Clinical evaluation of EliA™ Calprotectin Fecal calprotectin in children Stool extraction kits in comparison Screening for celiac disease ImmunoDiagnostics | Journal No. 7. 2013 Autoimmune Gastrointestinal Diseases discussed in Freiburg at the 8th EliA Symposium From May 26 to 27 Phadia GmbH, now part of Thermo Fisher Scien- tific, organized the 8th scientific symposium in Freiburg. More than 200 guests from Europe, Asia CONTENTS and North America came 3 Analytical and clinical evaluation of fecal calprotectin to listen to a full program of lectures which were held as marker of inflammatory bowel disease 4 Evaluation of a new method for calprotectin analysis in feces with by eight internationally renowned speakers, all experts Phadia 250 in the field. 5 Fecal calprotectin in healthy children from 0 to 4 years 6 The New Assay Fecal Calprotectin in Random Access: What Changes for The chairman Prof. Ingvar Bjarnason from the King’s the Laboratory College Hospital in London led through the morning 7 First results on project “Calprotectin analysed with two methods held against clinical data” session which was dedicated to inflammatory bowel 8 Evaluation of EliA Test for the measure of fecal calprotectin levels diseases and the diagnostic marker fecal calprotectin. -

Viagra Purchase
Current Evidence in Allergic Rhinitis Brought to you by the ARS/AAOA Education Committees Chair Education Committee ARS: Jeremiah A. Alt MD PhD FARS FACS Project Co-Director AAOA: Kristin Seiberling MD Project Co-Director: Elina Toskala MD PhD MBA Contributing Authors American Rhinologic Society: Garret Choby MD, Anthony Del Signore MD, Carrie Flanagan MD, Wayne Hsueh MD, Ian Humphreys MD, Chris Ito MD, Sandra Lin MD, Mike Marino MD, Edward McCoul MD, Bobby Tajudeen MD, Arthur Wu MD, Mike Yim MD American Academy of Otolaryngic Allergy: Douglas Anderson MD, Dole Baker MD, Chris Brook MD, Melissa Hertler MD, Mona Patadia MD, Christopher Vickery MD Manuscript ▶ There is a wide variety in the type and quality of the growing literature on allergic rhinitis (AR) ▶ The International Consensus Statement on Allergy and Rhinology: Allergic Rhinitis (ICAR:AR) was developed to summarize the best evidence relating to AR. ▶ More than 100 international authors evaluated the evidence using a structured review process. Section I pg. 113 Introduction ▶ There is a wide variety in the type and quality of the growing literature on allergic rhinitis (AR) ▶ The International Consensus Statement on Allergy and Rhinology: Allergic Rhinitis (ICAR:AR) was developed to summarize the best evidence relating to AR. ▶ More than 100 international authors evaluated the evidence using a structured review process. Introduction ▶ This document summarizes findings of meta-analyses and other systematic reviews to provide recommendations based on the best AR evidence ▶ High value placed on strength of evidence ▶ Similar to the 2016 International Consensus Statement on Allergy and Rhinology: Rhinosinusitis (ICAR:RS), this is not a clinical practice guideline or meta-analysis ▶ Practitioners are able to use this evidence-based knowledge to provide support for treatment Section II Methods II.A. -

Immunocap® Rapid
SKUP Scandinavian evaluation of laboratory equipment for primary health care ImmunoCAP Rapid A near-patient test for qualitative detection of specific IgE antibodies against inhalation allergens in human whole blood manufactured by Phadia AB, now Thermo Fisher Scientific Inc, Uppsala, Sweden Report from an evaluation under standardised and optimal conditions and in primary health care organised by SKUP Evaluated at the request of Phadia, Denmark Hillerød, Phone +45 4829 4176 SKUP in Denmark, Dept.KBA, Hillerød Hospital, 3400 SKUP/2013/68 The report was written by SKUP, 2011. Main authors were Esther Jensen and Stine Beenfeldt Weber, SKUP in Denmark. ImmunoCAP Rapid Table of contents Table of contents TABLE OF CONTENTS................................................................................................................ 3 1. SUMMARY.................................................................................................................................. 4 2. ABBREVIATIONS ..................................................................................................................... 8 3. QUALITY GOALS ..................................................................................................................... 9 3.1. ANALYTICAL QUALITY GOALS .................................................................................................. 9 3.2. EVALUATION OF USER -FRIENDLINESS .................................................................................... 11 3.3. SKUP’ S QUALITY GOALS IN THIS EVALUATION -

Immunodiagnostics Journal
Journal No. 1. 2016 Scientific news, opinions and reports ImmunoJournalDiagnostics 12th Dresden Symposium on Autoantibodies: EASI (European Autoimmunity Standardization Initiative) Session on Celiac Disease The EASI session at the 12th Dresden Symposium on Autoantibodies took place on September 25, 2015. More than 200 delegates attended the two hour symposium, chaired by Professor Markku Mäki and Dr Eckart Mummert, to listen to experts presenting around the identification and management of celiac disease, and the role of a gluten-free diet on IgA mediated disease. A summary of each presentation is included in this volume of the ImmunoDiagnostics Journal. Serology and the diagnosis of celiac disease Early diet and the development of celiac disease The influence of a gluten-free diet on IgA mediated disease ImmunoDiagnostics | Journal No. 1. 2016 New Insights in the Identification of Celiac Disease CONTENTS 12th Dresden Symposium on Autoantibodies: EASI Session On September 25, 2015, in the EASI session at the 12th 3 Overview of celiac disease and the special Dresden Symposium on Autoantibodies, experts from challenges in diagnostics across Europe presented thought provoking lectures around M Mäki a central theme of celiac disease. Over 200 delegates School on Medicine, University of Tampere, Finland attended and, through insightful questions, engaged in 5 Influence of diet in the firstear y of life on the the session. One of the Chairmen, Prof Mäki from the development of celiac disease. Major results of University of Tampere, Finland, started the session with the PreventCD study an overview of celiac disease and the challenges of Ilma R Korponay-Szabó University of Debrecen and Heim Pál Children’s Hospital diagnostics. -

Immunocap Specific
TM • ImmunoCAP Specific IgE Calibrator Strip 0-100 (Art No 10-9459-01: for 5 calibration • Washing Solution (Art No 10-9202-01: 2 x 5 l) ImmunoCAP Specific IgE curves) • Washing Solution Additive, 2 x 86 ml • ImmunoCAP Specific IgE Curve Control Strip (CC-1 and CC-2) (Art No 10-9312-01: • Washing Solution Concentrate, 2 x 400 ml Fluoroenzymeimmunoassay 5 x 3 sets of curve control) • ImmunoCAP Specific IgE Control L (Art No 10-9528-01: for 6 x 4 determinations) Calibrator Range 0-100 kU/l • ImmunoCAP Specific IgE Anti-IgE (a_IgE) (Art No 14-4417-01: carriers of 16 • ImmunoCAP Specific IgE Control M (Art No 10-9529-01: for 6 x 4 determinations) Directions for Use 52-5291-EN/09 ImmunoCAP) • ImmunoCAP Specific IgE Control H (Art No 10-9530-01: for 6 x 4 determinations) • ImmunoCAP Allergen (See Product catalogue: carriers of 16 or 10 ImmunoCAP) • ImmunoCAP Specific IgE Negative Control (Art No 10-9445-01: for 6 x 4 • ImmunoCAP Total IgE Low Range (low) (Art No 14-4497-35: for 48 determinations) determinations) INTENDED USE • ImmunoCAP Phadiatop (phad) (Art No 14-4405-35: for 48 determinations) ImmunoCAP Specific IgE is an in vitro test system for the quantitative measurement of allergen • ImmunoCAP Phadiatop Infant (phinf) (Art No 14-4510-35: for 48 determinations) Reagents for Phadia 2500 and Phadia 5000 specific IgE in human serum or plasma. It is intended for in vitro diagnostic use as an aid in • Development Solution (Art No 10-9441-01: for 6 x 200 determinations; Art No • ImmunoCAP Specific IgE Conjugate 400 (Art No 10-9310-01: for 6 x 400 the clinical diagnosis of IgE mediated allergic disorders in conjunction with other clinical 10-9440-01: for 6 x 315 determinations) determinations) findings, and is to be used in clinical laboratories. -

Product Catalog 2020 - 2 - Table of Contents
Product Catalog 2020 - 2 - Table of Contents Table Page Introduction 5 Available assays 6 Overview of instruments and assays 7 Allergy and asthma ImmunoCAP™ Total IgE 8 ImmunoCAP™ Total IgE Low Range 9 ImmunoCAP™ Specific IgE 10 ImmunoCAP™ Phadiatop™ / Phadiatop™ Infant 11 ImmunoCAP™ Specific IgG 12 ImmunoCAP™ Specific IgG4 13 ImmunoCAP™ Specific IgA 14 ImmunoCAP™ ECP 15 ImmunoCAP™ Tryptase 16 ImmunoCAP™ Allergen components 17 ImmunoCAP™ Allergens 19 ImmunoCAP™ Antigens 33 ImmunoCAP™ Research Use Only Products 34 ImmunoCAP™ Rapid 35 ImmunoCAP™ ISAC 36 Autoimmunity EliA™ 39 Phadia™ Laboratory Systems Phadia™ 5000 48 System reagents, Accessories and Software Phadia™ 2500 49 System reagents, Accessories and Software Phadia™ 1000 50 System reagents, Accessories and Software Phadia™ 250 51 System reagents, Accessories and Software Phadia™ 200 52 System reagents, Accessories and Software Phadia™ 100 53 System reagents, Accessories and Software Quality Club™ 54 Specifications 55 ImmunoCAP™ and EliA™ All products mentioned herein are produced according to ISO 13485:2003. - 3 - - 4 - Introduction About us ImmunoDiagnostics – the global leader in allergy and autoimmunity diagnostics. As the ImmunoDiagnostics experts within Thermo Fisher Scientific, we work to significantly improve the management of allergy, asthma and autoimmune diseases. We do this by providing healthcare professionals with diagnostic technologies and clinical expertise. This results in better healthcare and quality of life for millions of patients and their families. For more information, please visit www.thermoscientific.com/phadia. Thermo Fisher Scientific Inc. is the world leader in serving science, with annual revenue exceeding $25 billion and more than 75,000 colleagues worldwide. Our mission is to enable our customers to make the world healthier, cleaner and safer. -

Report of 3 Cases of Anaphylaxis Post Pfizer Bnt162b2 Vaccination
Case Report Pseudo-Anaphylactic Reactions to Pfizer BNT162b2 Vaccine: Report of 3 Cases of Anaphylaxis Post Pfizer BNT162b2 Vaccination Xin Rong Lim 1,* , Bernard Pui Leung 1,2, Carol Yee Leng Ng 3, Justina Wei Lynn Tan 1, Grace Yin Lai Chan 1, Chien Mei Loh 3, Gwendolyn Li Xuan Tan 3, Valerie Hui Hian Goh 3, Lok To Wong 3, Chong Rui Chua 3, Sze Chin Tan 1, Samuel Shang Ming Lee 1, Hwee Siew Howe 1, Bernard Yu Hor Thong 1 and Khai Pang Leong 1,3 1 Department of Rheumatology, Allergy and Immunology, Tan Tock Seng Hospital, 11 Jalan Tan Tock Seng, Singapore 308433, Singapore; [email protected] (B.P.L.); [email protected] (J.W.L.T.); [email protected] (G.Y.L.C.); [email protected] (S.C.T.); [email protected] (S.S.M.L.); [email protected] (H.S.H.); [email protected] (B.Y.H.T.); [email protected] (K.P.L.) 2 Health and Social Sciences, Singapore Institute of Technology, Singapore 138683, Singapore 3 Clinical Immunology Laboratory, Tan Tock Seng Hospital, Singapore 308433, Singapore; [email protected] (C.Y.L.N.); [email protected] (C.M.L.); [email protected] (G.L.X.T.); [email protected] (V.H.H.G.); [email protected] (L.T.W.); [email protected] (C.R.C.) * Correspondence: [email protected]; Tel.: +(65)-6357-7822; Fax: +(65)-6357-2686 Abstract: Anaphylactic reactions were observed after Singapore’s national coronavirus disease 2019 Citation: Lim, X.R.; Leung, B.P.; Ng, (COVID-19) vaccination programme started in December 2020. -

Clinically Relevant Discrepancies Between Different Rheumatoid Factor
Clin Chem Lab Med 2018; aop Willem J.J. Falkenburg*, Helen J. von Richthofen, Jana Koers, Cas Weykamp, Marco W.J. Schreurs, Liesbeth E. Bakker-Jonges, Inez-Anne Haagen, Willem F. Lems, Dörte Hamann, Dirkjan van Schaardenburg and Theo Rispens* Clinically relevant discrepancies between different rheumatoid factor assays https://doi.org/10.1515/cclm-2017-0988 virtually all RF responses reacted with both targets, Received November 3, 2017; accepted December 14, 2017 although levels were still variable. Abstract Conclusions: The use of RF assays that differ in technique and target antigen, together with the different specificities Background: Accurate measurements of rheumatoid of RF responses, leads to discrepancies in RF status and factors (RFs), autoantibodies binding IgG, are important levels. This has important consequences for patient care for diagnosing rheumatoid arthritis (RA) and for predict- if RA diagnosis and disease progression assessments are ing disease course. Worldwide, various RF assays are based on RF test results. being used that differ in technique and target antigens. We Keywords: arthralgia; assays; rabbit IgG; rheumatoid studied whether assay choice leads to clinically important arthritis; rheumatoid factor; target antigen. discrepancies in RF status and level. Methods: RF measurements using four commercial RF assays were compared in 32 RF+ samples. Using enzyme- linked immunosorbent assays (ELISAs), the influence Introduction of the target antigen source – human IgG (hIgG) versus rabbit IgG (rIgG) – on measured RF levels was investigated Rheumatoid factors (RFs) are autoantibodies that bind in arthralgia patients and RA patients. to epitopes within the constant region (Fc) of IgG. The Results: Substantial discrepancies were found between first evidence for their existence was found in 1937 when RF levels measured in the four commercial assays. -

Product Catalog 2020 - 2 - Table of Contents
Product Catalog 2020 - 2 - Table of Contents Table Page Introduction 5 Available assays 6 Overview of instruments and assays 7 Allergy and asthma ImmunoCAP™ Total IgE 8 ImmunoCAP™ Total IgE Low Range 9 ImmunoCAP™ Specific IgE 10 ImmunoCAP™ Phadiatop™ / Phadiatop™ Infant 11 ImmunoCAP™ Specific IgG 12 ImmunoCAP™ Specific IgG4 13 ImmunoCAP™ Specific IgA 14 ImmunoCAP™ ECP 15 ImmunoCAP™ Tryptase 16 ImmunoCAP™ Allergen components 17 ImmunoCAP™ Allergens 19 ImmunoCAP™ Antigens 33 ImmunoCAP™ Research Use Only Products 34 ImmunoCAP™ Rapid 35 ImmunoCAP™ ISAC 36 Autoimmunity EliA™ 39 Phadia™ Laboratory Systems Phadia™ 5000 48 System reagents, Accessories and Software Phadia™ 2500 49 System reagents, Accessories and Software Phadia™ 1000 50 System reagents, Accessories and Software Phadia™ 250 51 System reagents, Accessories and Software Phadia™ 200 52 System reagents, Accessories and Software Phadia™ 100 53 System reagents, Accessories and Software Quality Club™ 54 Specifications 55 ImmunoCAP™ and EliA™ All products mentioned herein are produced according to ISO 13485:2003. - 3 - - 4 - Introduction About us ImmunoDiagnostics – the global leader in allergy and autoimmunity diagnostics. As the ImmunoDiagnostics experts within Thermo Fisher Scientific, we work to significantly improve the management of allergy, asthma and autoimmune diseases. We do this by providing healthcare professionals with diagnostic technologies and clinical expertise. This results in better healthcare and quality of life for millions of patients and their families. For more information, please visit www.thermoscientific.com/phadia. Thermo Fisher Scientific Inc. is the world leader in serving science, with annual revenue exceeding $25 billion and more than 75,000 colleagues worldwide. Our mission is to enable our customers to make the world healthier, cleaner and safer. -

510(K) Substantial Equivalence Determination Decision Memorandum Assay Only Template
510(k) SUBSTANTIAL EQUIVALENCE DETERMINATION DECISION SUMMARY A. 510(k) Number: K190710 B. Purpose for Submission: Revision of assay, migration to new instrument C. Measurand: Human IgG autoantibodies to: • U1RNP/RNP70 (A/C) • SS-A/Ro (52, 60 kDa) • SS-B/La • Centromere B (CENP-B) • Scl-70 (topoisomerase I) • Jo-1 (histidyl tRNA synthetase) • SmD3 peptide D. Type of Test: Automated qualitative multianalyte solid-phase immunoassay E. Applicant: Phadia US, Inc. F. Proprietary and Established Names: Phadia EliA SymphonyS Immunoassay G. Regulatory Information: 1. Regulation section: 21 CFR §866.5100– Antinuclear antibody immunological test system 2. Classification: Class II 1 3. Product code: LLL ‒ Extractable Antinuclear Antibody, Antigen And Control 4. Panel: Immunology (82) H. Intended Use: 1. Intended use: EliA SymphonyS is intended for the in vitro, qualitative measurement of antinuclear IgG antibodies in human serum and plasma (Li-heparin, EDTA). EliA SymphonyS is based on human recombinant U1RNP (RNP 70, A, C), SS-A/Ro (60 kDa, 52 kDa), SSB/La, Centromere B, Scl-70, Jo-1 proteins and a synthetic SmD3 peptide as antigen and is useful as an aid in the clinical diagnosis of patients with systemic lupus erythematosus (SLE), mixed connective tissue disease (MCTD), Sjögren's syndrome, scleroderma and polymyositis/dermatomyositis, in conjunction with other laboratory and clinical findings. EliA SymphonyS uses the EliA IgG method on the instrument Phadia 250. EliA SymphonyS is intended for the in vitro, qualitative measurement of antinuclear IgG antibodies in human serum and plasma (Li-heparin, EDTA). EliA SymphonyS is based on human recombinant U1RNP (RNP 70, A, C), SS-A/Ro (60 kDa, 52 kDa), SSB/La, Centromere B, Scl-70, Jo-1 proteins and a synthetic SmD3 peptide as antigen and is useful as an aid in the clinical diagnosis of patients with systemic lupus erythematosus (SLE), mixed connective tissue disease (MCTD), Sjögren's syndrome, scleroderma and polymyositis/dermatomyositis, in conjunction with other laboratory and clinical findings.