Table of Contents (PDF)
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Appendix 2. Significantly Differentially Regulated Genes in Term Compared with Second Trimester Amniotic Fluid Supernatant
Appendix 2. Significantly Differentially Regulated Genes in Term Compared With Second Trimester Amniotic Fluid Supernatant Fold Change in term vs second trimester Amniotic Affymetrix Duplicate Fluid Probe ID probes Symbol Entrez Gene Name 1019.9 217059_at D MUC7 mucin 7, secreted 424.5 211735_x_at D SFTPC surfactant protein C 416.2 206835_at STATH statherin 363.4 214387_x_at D SFTPC surfactant protein C 295.5 205982_x_at D SFTPC surfactant protein C 288.7 1553454_at RPTN repetin solute carrier family 34 (sodium 251.3 204124_at SLC34A2 phosphate), member 2 238.9 206786_at HTN3 histatin 3 161.5 220191_at GKN1 gastrokine 1 152.7 223678_s_at D SFTPA2 surfactant protein A2 130.9 207430_s_at D MSMB microseminoprotein, beta- 99.0 214199_at SFTPD surfactant protein D major histocompatibility complex, class II, 96.5 210982_s_at D HLA-DRA DR alpha 96.5 221133_s_at D CLDN18 claudin 18 94.4 238222_at GKN2 gastrokine 2 93.7 1557961_s_at D LOC100127983 uncharacterized LOC100127983 93.1 229584_at LRRK2 leucine-rich repeat kinase 2 HOXD cluster antisense RNA 1 (non- 88.6 242042_s_at D HOXD-AS1 protein coding) 86.0 205569_at LAMP3 lysosomal-associated membrane protein 3 85.4 232698_at BPIFB2 BPI fold containing family B, member 2 84.4 205979_at SCGB2A1 secretoglobin, family 2A, member 1 84.3 230469_at RTKN2 rhotekin 2 82.2 204130_at HSD11B2 hydroxysteroid (11-beta) dehydrogenase 2 81.9 222242_s_at KLK5 kallikrein-related peptidase 5 77.0 237281_at AKAP14 A kinase (PRKA) anchor protein 14 76.7 1553602_at MUCL1 mucin-like 1 76.3 216359_at D MUC7 mucin 7, -

The Role of Components of the Chromatin Modification Machinery in Carcinogenesis of Clear Cell Carcinoma of the Ovary (Review)
ONCOLOGY LETTERS 2: 591-597, 2011 The role of components of the chromatin modification machinery in carcinogenesis of clear cell carcinoma of the ovary (Review) HIROSHI SHIGETOMI, AKIRA OONOGI, TAIHEI TSUNEMI, YASUHITO TANASE, YOSHIHIKO YAMADA, HIROTAKA KAJIHARA, YORIKO YOSHIZAWA, NAOTO FURUKAWA, SHOJI HARUTA, SHOZO YOSHIDA, TOSHIYUKI SADO, HIDEKAZU OI and HIROSHI KOBAYASHI Department of Obstetrics and Gynecology, Nara Medical University, Nara, Japan Received January 21, 2011; Accepted April 27, 2011 DOI: 10.3892/ol.2011.316 Abstract. Recent data have provided information regarding 6. A marked resemblance between CCC and ccRCC the profiles of clear cell carcinoma of the ovary (CCC) with 7. Conclusions adenine-thymine rich interactive domain 1A (ARID1A) muta- tions. The purpose of this review was to summarize current 1. Introduction knowledge regarding the molecular mechanisms involved in CCC tumorigenesis and to describe the central role played Epithelial ovarian cancer (EOC) is the most lethal gyne- by the aberrant chromatin remodeling. The present article cologic malignancy worldwide. Epidemiology calculations reviews the English-language literature for biochemical of lifetime risk for EOC are that 1 in 55 women is likely to studies on the ARID1A mutation and chromatin remodeling develop EOC during their lifetime (1). Since EOC is more in CCC. ARID1A is responsible for directing the SWI/SNF likely to be advanced stage with unfavorable tumor biology, complex to target promoters and regulates the transcription of there are serious limitations to the surgical and oncological certain genes by altering the chromatin structure around those treatment available. Therefore, it is crucial to determine the genes. The mutation spectrum of ARID1A was enriched for earliest possible diagnosis. -

Development and Validation of a Protein-Based Risk Score for Cardiovascular Outcomes Among Patients with Stable Coronary Heart Disease
Supplementary Online Content Ganz P, Heidecker B, Hveem K, et al. Development and validation of a protein-based risk score for cardiovascular outcomes among patients with stable coronary heart disease. JAMA. doi: 10.1001/jama.2016.5951 eTable 1. List of 1130 Proteins Measured by Somalogic’s Modified Aptamer-Based Proteomic Assay eTable 2. Coefficients for Weibull Recalibration Model Applied to 9-Protein Model eFigure 1. Median Protein Levels in Derivation and Validation Cohort eTable 3. Coefficients for the Recalibration Model Applied to Refit Framingham eFigure 2. Calibration Plots for the Refit Framingham Model eTable 4. List of 200 Proteins Associated With the Risk of MI, Stroke, Heart Failure, and Death eFigure 3. Hazard Ratios of Lasso Selected Proteins for Primary End Point of MI, Stroke, Heart Failure, and Death eFigure 4. 9-Protein Prognostic Model Hazard Ratios Adjusted for Framingham Variables eFigure 5. 9-Protein Risk Scores by Event Type This supplementary material has been provided by the authors to give readers additional information about their work. Downloaded From: https://jamanetwork.com/ on 10/02/2021 Supplemental Material Table of Contents 1 Study Design and Data Processing ......................................................................................................... 3 2 Table of 1130 Proteins Measured .......................................................................................................... 4 3 Variable Selection and Statistical Modeling ........................................................................................ -

Human Induced Pluripotent Stem Cell–Derived Podocytes Mature Into Vascularized Glomeruli Upon Experimental Transplantation
BASIC RESEARCH www.jasn.org Human Induced Pluripotent Stem Cell–Derived Podocytes Mature into Vascularized Glomeruli upon Experimental Transplantation † Sazia Sharmin,* Atsuhiro Taguchi,* Yusuke Kaku,* Yasuhiro Yoshimura,* Tomoko Ohmori,* ‡ † ‡ Tetsushi Sakuma, Masashi Mukoyama, Takashi Yamamoto, Hidetake Kurihara,§ and | Ryuichi Nishinakamura* *Department of Kidney Development, Institute of Molecular Embryology and Genetics, and †Department of Nephrology, Faculty of Life Sciences, Kumamoto University, Kumamoto, Japan; ‡Department of Mathematical and Life Sciences, Graduate School of Science, Hiroshima University, Hiroshima, Japan; §Division of Anatomy, Juntendo University School of Medicine, Tokyo, Japan; and |Japan Science and Technology Agency, CREST, Kumamoto, Japan ABSTRACT Glomerular podocytes express proteins, such as nephrin, that constitute the slit diaphragm, thereby contributing to the filtration process in the kidney. Glomerular development has been analyzed mainly in mice, whereas analysis of human kidney development has been minimal because of limited access to embryonic kidneys. We previously reported the induction of three-dimensional primordial glomeruli from human induced pluripotent stem (iPS) cells. Here, using transcription activator–like effector nuclease-mediated homologous recombination, we generated human iPS cell lines that express green fluorescent protein (GFP) in the NPHS1 locus, which encodes nephrin, and we show that GFP expression facilitated accurate visualization of nephrin-positive podocyte formation in -

Arid1a Is Essential for Intestinal Stem Cells Through Sox9 Regulation
Arid1a is essential for intestinal stem cells through Sox9 regulation Yukiko Hiramatsua, Akihisa Fukudaa,1, Satoshi Ogawaa, Norihiro Gotoa, Kozo Ikutaa, Motoyuki Tsudaa, Yoshihide Matsumotoa, Yoshito Kimuraa, Takuto Yoshiokaa, Yutaka Takadaa, Takahisa Marunoa, Yuta Hanyua, Tatsuaki Tsuruyamab, Zhong Wangc, Haruhiko Akiyamad, Shigeo Takaishie, Hiroyuki Miyoshif, Makoto Mark Taketof, Tsutomu Chibag, and Hiroshi Senoa aDepartment of Gastroenterology and Hepatology, Kyoto University Graduate School of Medicine, 606-8507 Kyoto, Japan; bClinical Bioresource Center, Kyoto University Hospital, 606-8507 Kyoto, Japan; cDepartment of Cardiac Surgery, Cardiovascular Research Center, University of Michigan, Ann Arbor, MI 48109; dDepartment of Orthopaedics, Gifu University, 501-1194 Gifu, Japan; eLaboratory for Malignancy Control Research (DSK project), Medical Innovation Center, Kyoto University Graduate School of Medicine, 606-8507 Kyoto, Japan; fDivision of Experimental Therapeutics, Kyoto University Graduate School of Medicine, Yoshida-Konoe-cho, Sakyo-ku, 606-8506 Kyoto, Japan; and gKansai Electric Power Hospital, 553-0003 Osaka, Japan Edited by Hans Clevers, Hubrecht Institute, Utrecht, The Netherlands, and approved December 13, 2018 (received for review March 21, 2018) Inactivating mutations of Arid1a, a subunit of the Switch/sucrose tion (6, 7). In addition, a recent study showed that deletion of nonfermentable chromatin remodeling complex, have been Arid1a in the intestines induces colon cancer in mice (8). How- reported in multiple human cancers. Intestinal deletion of Arid1a ever, the functional role of Arid1a in intestinal homeostasis and has been reported to induce colorectal cancer in mice; however, its its underlying molecular mechanisms remain unknown. functional role in intestinal homeostasis remains unclear. We in- Recently, studies with transgenic and knockout mice have vestigated the functional role of Arid1a in intestinal homeostasis elucidated the molecular mechanisms underlying the develop- in mice. -

Atf1 Arid3a Atf2
TFs Datasets Sequence logos of information models showing cofactor motifs Top 500 peaks (FOXA motif) HepG2, Stanford ARID3A (optimal IDR thresholded) Top 5000 peaks (FOXA motif) All 14818 peaks (IRF motif) K562, HMS ATF1 (optimal IDR thresholded) Top 2500 peaks (SP motif) H1-hESC, HAIB ATF2 Protocol: v042211.1 (optimal IDR thresholded) All 1860 peaks (USF motif) GM12878, HAIB Protocol: PCR1x (SPP obtained from Factorbook) All 1670 peaks (SP motif) GM12878, HAIB Protocol: PCR1x (optimal IDR thresholded) 1550 peaks (USF motif), after masking the SP motif All 4803 peaks (SP motif) H1-hESC, HAIB Protocol: v041610.2 (optimal IDR thresholded) 4697 peaks (USF motif), after masking the SP motif ATF3 All 3284 peaks (SP motif) HepG2, HAIB Protocol: v041610.1 (optimal IDR thresholded) 3209 peaks (USF motif), after masking the SP motif All 11098 peaks (SP motif) K562, HAIB, Replicate 1 Protocol: v041610.1 (raw peaks) All 1232 peaks (SP motif) K562, HMS (optimal IDR thresholded) 1210 peaks (USF motif), after masking the SP motif All 4448 peaks (AP1 motif) BAF155/ HeLa-S3, Stanford SMARCC1 (SPP obtained from Factorbook) 1342 peaks (AP1 motif), after using masking BAF170/ HeLa-S3, Stanford SMARCC2 (SPP obtained from Factorbook) All 17809 peaks (IRF motif) GM12878, HAIB Protocol: PCR1x (optimal IDR thresholded) 17321 peaks (RUNX motif), after masking the IRF motif BCL11A All 2513 peaks (SOX2-OCT4 motif) H1-hESC, HAIB Protocol: PCR1x (optimal IDR thresholded) All 7707 peaks (AP1 motif), after masking noise A549, HAIB Protocol: v042211.1 BCL3 Treatment: -

Supplement. Transcriptional Factors (TF), Protein Name and Their Description Or Function
Supplement. Transcriptional factors (TF), protein name and their description or function. TF Protein name TF description/function ARID3A AT rich interactive domain 3A (BRIGHT-like) This gene encodes a member of the ARID (AT-rich interaction domain) family of DNA binding proteins. ATF4 Activating Transcription Factor 4 Transcriptional activator. Binds the cAMP response element (CRE) (consensus: 5-GTGACGT[AC][AG]-3), a sequence present in many viral and cellular promoters. CTCF CCCTC-Binding Factor Chromatin binding factor that binds to DNA sequence specific sites. Involved in transcriptional regulation by binding to chromatin insulators and preventing interaction between promoter and nearby enhancers and silencers. The protein can bind a histone acetyltransferase (HAT)-containing complex and function as a transcriptional activator or bind a histone deacetylase (HDAC)-containing complex and function as a transcriptional repressor. E2F1-6 E2F transcription factors 1-6 The protein encoded by this gene is a member of the E2F family of transcription factors. The E2F family plays a crucial role in the control of cell cycle and action of tumor suppressor proteins and is also a target of the transforming proteins of small DNA tumor viruses. The E2F proteins contain several evolutionally conserved domains found in most members of the family. These domains include a DNA binding domain, a dimerization domain which determines interaction with the differentiation regulated transcription factor proteins (DP), a transactivation domain enriched in acidic amino acids, and a tumor suppressor protein association domain which is embedded within the transactivation domain. EBF1 Transcription factor COE1 EBF1 has been shown to interact with ZNF423 and CREB binding proteins. -

Computational Discovery of Transcription Factors Associated with Drug Response
OPEN The Pharmacogenomics Journal (2016) 16, 573–582 www.nature.com/tpj ORIGINAL ARTICLE Computational discovery of transcription factors associated with drug response C Hanson1, J Cairns2, L Wang2 and S Sinha3 This study integrates gene expression, genotype and drug response data in lymphoblastoid cell lines with transcription factor (TF)-binding sites from ENCODE (Encyclopedia of Genomic Elements) in a novel methodology that elucidates regulatory contexts associated with cytotoxicity. The method, GENMi (Gene Expression iN the Middle), postulates that single-nucleotide polymorphisms within TF-binding sites putatively modulate its regulatory activity, and the resulting variation in gene expression leads to variation in drug response. Analysis of 161 TFs and 24 treatments revealed 334 significantly associated TF–treatment pairs. Investigation of 20 selected pairs yielded literature support for 13 of these associations, often from studies where perturbation of the TF expression changes drug response. Experimental validation of significant GENMi associations in taxanes and anthracyclines across two triple-negative breast cancer cell lines corroborates our findings. The method is shown to be more sensitive than an alternative, genome-wide association study-based approach that does not use gene expression. These results demonstrate the utility of GENMi in identifying TFs that influence drug response and provide a number of candidates for further testing. The Pharmacogenomics Journal (2016) 16, 573–582; doi:10.1038/tpj.2015.74; published online 27 October 2015 INTRODUCTION regulatory activities are associated with cellular response to The field of pharmacogenetics aims to understand the relationship cytotoxic treatments (Figure 1a), with the expectation that in the between individual variation at the genetic level and variation in future the response may be manipulated by intervening with the cellular or physiological response to a drug. -

A Microarray Platform-Independent Classification Tool for Cell of Origin Class Allows Comparative Analysis of Gene Expression in Diffuse Large B-Cell Lymphoma
A Microarray Platform-Independent Classification Tool for Cell of Origin Class Allows Comparative Analysis of Gene Expression in Diffuse Large B-cell Lymphoma Matthew A. Care1,2, Sharon Barrans3, Lisa Worrillow3, Andrew Jack3, David R. Westhead2*, Reuben M. Tooze1,3* 1 Section of Experimental Haematology, Leeds Institute of Molecular Medicine, University of Leeds, Leeds, United Kingdom, 2 Bioinformatics Group, Institute of Molecular and Cellular Biology, University of Leeds, Leeds, United Kingdom, 3 Haematological Malignancy Diagnostic Service (HMDS), St. James’s Institute of Oncology, Leeds, United Kingdom Abstract Cell of origin classification of diffuse large B-cell lymphoma (DLBCL) identifies subsets with biological and clinical significance. Despite the established nature of the classification existing studies display variability in classifier implementation, and a comparative analysis across multiple data sets is lacking. Here we describe the validation of a cell of origin classifier for DLBCL, based on balanced voting between 4 machine-learning tools: the DLBCL automatic classifier (DAC). This shows superior survival separation for assigned Activated B-cell (ABC) and Germinal Center B-cell (GCB) DLBCL classes relative to a range of other classifiers. DAC is effective on data derived from multiple microarray platforms and formalin fixed paraffin embedded samples and is parsimonious, using 20 classifier genes. We use DAC to perform a comparative analysis of gene expression in 10 data sets (2030 cases). We generate ranked meta-profiles of genes showing consistent class-association using $6 data sets as a cut-off: ABC (414 genes) and GCB (415 genes). The transcription factor ZBTB32 emerges as the most consistent and differentially expressed gene in ABC-DLBCL while other transcription factors such as ARID3A, BATF, and TCF4 are also amongst the 24 genes associated with this class in all datasets. -

Repurposing of KLF5 Activates a Cell Cycle Signature During The
RESEARCH ARTICLE Repurposing of KLF5 activates a cell cycle signature during the progression from a precursor state to oesophageal adenocarcinoma Connor Rogerson1, Samuel Ogden1, Edward Britton1, The OCCAMS Consortium, Yeng Ang2,3*, Andrew D Sharrocks1* 1School of Biological Sciences, Faculty of Biology, Medicine and Health, University of Manchester, Manchester, United Kingdom; 2School of Medical Sciences, Faculty of Biology, Medicine and Health, University of Manchester, Manchester, United Kingdom; 3GI Science Centre, Salford Royal NHS FT, University of Manchester, Salford, United Kingdom Abstract Oesophageal adenocarcinoma (OAC) is one of the most common causes of cancer deaths. Barrett’s oesophagus (BO) is the only known precancerous precursor to OAC, but our understanding about the molecular events leading to OAC development is limited. Here, we have integrated gene expression and chromatin accessibility profiles of human biopsies and identified a strong cell cycle gene expression signature in OAC compared to BO. Through analysing associated chromatin accessibility changes, we have implicated the transcription factor KLF5 in the transition from BO to OAC. Importantly, we show that KLF5 expression is unchanged during this transition, but instead, KLF5 is redistributed across chromatin to directly regulate cell cycle genes specifically in OAC cells. This new KLF5 target gene programme has potential prognostic significance as high *For correspondence: levels correlate with poorer patient survival. Thus, the repurposing of KLF5 for novel regulatory [email protected] (YA); activity in OAC provides new insights into the mechanisms behind disease progression. andrew.d.sharrocks@manchester. ac.uk (ADS) Competing interests: The authors declare that no Introduction competing interests exist. Oesophageal cancer is the eighth most common cancer worldwide, and its 5-year survival rate of Funding: See page 18 15% makes it the sixth most-common cause of cancer-related death (Ferlay et al., 2015; Received: 24 March 2020 Pennathur et al., 2013). -

Interplay Between Cofactors and Transcription Factors in Hematopoiesis and Hematological Malignancies
Signal Transduction and Targeted Therapy www.nature.com/sigtrans REVIEW ARTICLE OPEN Interplay between cofactors and transcription factors in hematopoiesis and hematological malignancies Zi Wang 1,2, Pan Wang2, Yanan Li2, Hongling Peng1, Yu Zhu2, Narla Mohandas3 and Jing Liu2 Hematopoiesis requires finely tuned regulation of gene expression at each stage of development. The regulation of gene transcription involves not only individual transcription factors (TFs) but also transcription complexes (TCs) composed of transcription factor(s) and multisubunit cofactors. In their normal compositions, TCs orchestrate lineage-specific patterns of gene expression and ensure the production of the correct proportions of individual cell lineages during hematopoiesis. The integration of posttranslational and conformational modifications in the chromatin landscape, nucleosomes, histones and interacting components via the cofactor–TF interplay is critical to optimal TF activity. Mutations or translocations of cofactor genes are expected to alter cofactor–TF interactions, which may be causative for the pathogenesis of various hematologic disorders. Blocking TF oncogenic activity in hematologic disorders through targeting cofactors in aberrant complexes has been an exciting therapeutic strategy. In this review, we summarize the current knowledge regarding the models and functions of cofactor–TF interplay in physiological hematopoiesis and highlight their implications in the etiology of hematological malignancies. This review presents a deep insight into the physiological and pathological implications of transcription machinery in the blood system. Signal Transduction and Targeted Therapy (2021) ;6:24 https://doi.org/10.1038/s41392-020-00422-1 1234567890();,: INTRODUCTION by their ATPase subunits into four major families, including the Hematopoiesisisacomplexhierarchicaldifferentiationprocessthat SWI/SNF, ISWI, Mi-2/NuRD, and INO80/SWR1 families. -
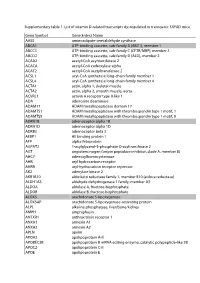
Supplementary Table 1. List of Vitamin D-Related Transcripts Dysregulated in Transgenic 5XFAD Mice
Supplementary table 1. List of vitamin D-related transcripts dysregulated in transgenic 5XFAD mice Gene Symbol Gene Entrez Name AASS aminoadipate-semialdehyde synthase ABCA1 ATP-binding cassette, sub-family A (ABC1), member 1 ABCC3 ATP-binding cassette, sub-family C (CFTR/MRP), member 3 ABCD2 ATP-binding cassette, sub-family D (ALD), member 2 ACAA2 acetyl-CoA acyltransferase 2 ACACA acetyl-CoA carboxylase alpha ACAT2 acetyl-CoA acetyltransferase 2 ACSL1 acyl-CoA synthetase long-chain family member 1 ACSL4 acyl-CoA synthetase long-chain family member 4 ACTA1 actin, alpha 1, skeletal muscle ACTA2 actin, alpha 2, smooth muscle, aorta ACVRL1 activin A receptor type II-like 1 ADA adenosine deaminase ADAM17 ADAM metallopeptidase domain 17 ADAMTS1 ADAM metallopeptidase with thrombospondin type 1 motif, 1 ADAMTS9 ADAM metallopeptidase with thrombospondin type 1 motif, 9 ADRA1B adrenoceptor alpha 1B ADRA1D adrenoceptor alpha 1D ADRB3 adrenoceptor beta 3 AEBP1 AE binding protein 1 AFP alpha-fetoprotein AGPAT2 1-acylglycerol-3-phosphate O-acyltransferase 2 AGT angiotensinogen (serpin peptidase inhibitor, clade A, member 8) AHCY adenosylhomocysteinase AHR aryl hydrocarbon receptor AHRR aryl-hydrocarbon receptor repressor AK2 adenylate kinase 2 AKR1B10 aldo-keto reductase family 1, member B10 (aldose reductase) ALDH1A3 aldehyde dehydrogenase 1 family, member A3 ALDOA aldolase A, fructose-bisphosphate ALDOB aldolase B, fructose-bisphosphate ALOX5 arachidonate 5-lipoxygenase ALOX5AP arachidonate 5-lipoxygenase-activating protein ALPL alkaline phosphatase,