Naujan Lake National Park Site Assessment Profile
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

China, in Spite of Much Forest Being Cut Down
A birdwatching tour to CCHHIINNAA,, HHOONNGG KKOONNGG && TTHHEE PPHHIILLIIPPPPIINNEESS 15.2 - 3.5 1987 Erling Jirle & Nils KjellŽn The birds in this checklist were seen on a trip to East Asia made by Nils KjellŽn and Erling Jirle from Lund, Sweden between February and May 1987. Our main purpose was to watch birds but of course also to have a look at the huge and fascinating China becoming easier to visit every year for individual travellers. Erling Jirle Lund December 1987 ©Erling Jirle 1987. Written on Macintosh Plus & Laserwriter. Second printing. January 1989. Web-version, November 1998. OOUURR RROOUUTTEE 15.2. Flight from Copenhagen - Amsterdam. Departure with Philippine Airlines' jumbo jet 4 p.m via Dubai (8 hrs) - Bangkok (6 hrs) to Manila (3 hrs). Lund - Manila took 26 hours in total. 17.2. Bird watching at American Cementary and Manila Bay (outside Pasay city). 18.2. Tour to Candaba swamps north of Manila. Unfortunately they were dry. 19.2. Bus to Malicboy 130 km south of Manila. Birdwatching in Quezon Natio- nal Park. 20.2. Birdwatching in Quezon N.P. 21.2. Visited the fishponds 3 km north of Malicboy. Quezon N.P. in the after- noon. Bus back to Manila in the evening. 22.2. Airbus 300 to Hong Kong in the morning. Birdwatching in Kowloon Park in the afternoon. 23.2. Visit to the Zoo. Invited to Dim Sum lunch. 24.2. Mai Po marshes the whole day. 25.2. Mai Po marshes. In the evening we crossed the chinese border. Train to Guangzhou (soft-seat). Slept outside a hotel (low-budget travelling). -

Mindoro East Coast Road Project
E1467 v 5 Public Disclosure Authorized Public Disclosure Authorized Public Disclosure Authorized Public Disclosure Authorized Table of Contents l'age I Executive Summary 1 I1 Project Description 4 Project Ra.tionale 4 Basic Project Information 5 Project Location 5 Description of Project Phases 6 111 Methodology Existing Erivironmental Condition Physical Environment Biological Environment Socio-Economic Environment IV Impact Assessment 23 Future Environmental Condition of the Project Area 23 Impacts Relating to Project Location 24 Impacts Relating to Project Construction 26 lmpacts Relating to Project Operation and Maintenance 30 V Environmental Management Plan 31 Environmental Monitoring Plan 39 VI ANNEXES Location Map Photographs along the Project Road Typical Section for flexible and rigid pavement Typical section of Bridge superstructure Provincial & Municipal Resolution Accountab~lityStatements Executive Summary Initial Environmental Examination (IEE) Mindoro East Coast Road Proiect Executive Summary A. Introduction The Environmental Impact Assessment (EIA) of the proposed Rehabilitationllmprovement of Mindoro East Coast Road Project (Bongabong - Roxas - Mansalay - Bulalacao - Magsaysay - San Jose Section) is presented in the form of an Initial Environmental Examination (IEE) to secure an Environmental Compliance Certificate (ECC) in accordance with the requirement of the revised rules and regulations of the Environmental Impact Statement System (EISS) embodied in .the Department of Environment and Natural Resources - Department Administrative Order (DENR-DAO) 96-37 Thus, this report covers the result of the said EIA that aims to confirm the environmental viability of implementing the proposed project. B. Project Description The 125.66 kilonieter Mindoro East Coast Road Project traverses the two provinces in the Island of Mindoro. It passes thru the municipalities of Bongabong, Roxas, Mansalay and Bulalacao in Oriental Mindoro and Magsaysay and San Jose in Occidental Mindoro. -

Bird List Column A: We Should Encounter (At Least a 90% Chance) Column B: May Encounter (About a 50%-90% Chance) Column C: Possible, but Unlikely (20% – 50% Chance)
THE PHILIPPINES Prospective Bird List Column A: we should encounter (at least a 90% chance) Column B: may encounter (about a 50%-90% chance) Column C: possible, but unlikely (20% – 50% chance) A B C Philippine Megapode (Tabon Scrubfowl) X Megapodius cumingii King Quail X Coturnix chinensis Red Junglefowl X Gallus gallus Palawan Peacock-Pheasant X Polyplectron emphanum Wandering Whistling Duck X Dendrocygna arcuata Eastern Spot-billed Duck X Anas zonorhyncha Philippine Duck X Anas luzonica Garganey X Anas querquedula Little Egret X Egretta garzetta Chinese Egret X Egretta eulophotes Eastern Reef Egret X Egretta sacra Grey Heron X Ardea cinerea Great-billed Heron X Ardea sumatrana Purple Heron X Ardea purpurea Great Egret X Ardea alba Intermediate Egret X Ardea intermedia Cattle Egret X Ardea ibis Javan Pond-Heron X Ardeola speciosa Striated Heron X Butorides striatus Yellow Bittern X Ixobrychus sinensis Von Schrenck's Bittern X Ixobrychus eurhythmus Cinnamon Bittern X Ixobrychus cinnamomeus Black Bittern X Ixobrychus flavicollis Black-crowned Night-Heron X Nycticorax nycticorax Western Osprey X Pandion haliaetus Oriental Honey-Buzzard X Pernis ptilorhynchus Barred Honey-Buzzard X Pernis celebensis Black-winged Kite X Elanus caeruleus Brahminy Kite X Haliastur indus White-bellied Sea-Eagle X Haliaeetus leucogaster Grey-headed Fish-Eagle X Ichthyophaga ichthyaetus ________________________________________________________________________________________________________ WINGS ● 1643 N. Alvernon Way Ste. 109 ● Tucson ● AZ ● 85712 ● www.wingsbirds.com -

Notas Breves FOOD HABITS of the ALPINE SWIFT on TWO
Ardeola 56(2), 2009, 259-269 Notas Breves FOOD HABITS OF THE ALPINE SWIFT ON TWO CONTINENTS: INTRA- AND INTERSPECIFIC COMPARISONS DIETA DEL VENCEJO REAL EN DOS CONTINENTES: ANÁLISIS COMPARATIVO INTRA E INTERESPECÍFICO Charles T. COLLINS* 1, José L. TELLA** and Brian D. COLAHAN*** SUMMARY.—The prey brought by alpine swifts Tachymarptis melba to their chicks in Switzerland, Spain and South Africa included a wide variety of arthropods, principally insects but also spiders. Insects com- prised 10 orders and 79 families, the Homoptera, Diptera and Hymenoptera being the most often con- sumed. The assessment of geographical variation in the diet was complicated by the great variability among the individual supplies of prey. Prey size varied between 1.3 and 29.6 mm, differing significantly in the median prey size of the three populations (5.12 - 8.81 mm) according to the intraspecific variation in size of alpine swifts. In an interspecific comparison, prey size correlated positively with body size in seven species of swifts. RESUMEN.—Las presas aportadas por vencejos reales Tachymarptis melba a sus pollos en Suiza, Es- paña y Sudáfrica incluyeron una amplia variedad de artrópodos, principalmente insectos pero también arañas. Entre los insectos, fueron identificados 10 órdenes y 79 familias, siendo Homoptera, Diptera e Hymenoptera los más consumidos. Las variaciones geográficas en la dieta se vieron ensombrecidas por una gran variabilidad entre los aportes individuales de presas. El tamaño de presa varió entre 1.3 y 29.6 mm, oscilando las medianas para cada población entre 5,12 y 8,81 mm. Los vencejos reales mostraron di- ferencias significativas en el tamaño de sus presas correspondiendo con su variación intraespecífica en tamaño. -

Conservation and Management of Eastern Big-Eared Bats a Symposium
Conservation and Management of Eastern Big-eared Bats A Symposium y Edited b Susan C. Loeb, Michael J. Lacki, and Darren A. Miller U.S. Department of Agriculture Forest Service Southern Research Station General Technical Report SRS-145 DISCLAIMER The use of trade or firm names in this publication is for reader information and does not imply endorsement by the U.S. Department of Agriculture of any product or service. Papers published in these proceedings were submitted by authors in electronic media. Some editing was done to ensure a consistent format. Authors are responsible for content and accuracy of their individual papers and the quality of illustrative materials. Cover photos: Large photo: Craig W. Stihler; small left photo: Joseph S. Johnson; small middle photo: Craig W. Stihler; small right photo: Matthew J. Clement. December 2011 Southern Research Station 200 W.T. Weaver Blvd. Asheville, NC 28804 Conservation and Management of Eastern Big-eared Bats: A Symposium Athens, Georgia March 9–10, 2010 Edited by: Susan C. Loeb U.S Department of Agriculture Forest Service Southern Research Station Michael J. Lacki University of Kentucky Darren A. Miller Weyerhaeuser NR Company Sponsored by: Forest Service Bat Conservation International National Council for Air and Stream Improvement (NCASI) Warnell School of Forestry and Natural Resources Offield Family Foundation ContEntS Preface . v Conservation and Management of Eastern Big-Eared Bats: An Introduction . 1 Susan C. Loeb, Michael J. Lacki, and Darren A. Miller Distribution and Status of Eastern Big-eared Bats (Corynorhinus Spp .) . 13 Mylea L. Bayless, Mary Kay Clark, Richard C. Stark, Barbara S. -

Characteristics, Threats and Management of Philippine Wetlands 필리핀 습지의 특성, 위협 및 관리
Journal of Wetlands Research ISSN 1229-6031 (Print) / ISSN 2384-0056 (Online) Vol. 18, No. 3, August 2016, pp. 250-261 DOI http://dx.doi.org/10.17663/JWR.2016.18.3.250 Characteristics, Threats and Management of Philippine Wetlands Shemelyn M. Sespeñe†・Marla Maniquiz-Redillas・Lee-Hyung Kim・Yun-wook Choo Department of Civil and Environmental Engineering, Kongju National University Cheonan City, Korea 필리핀 습지의 특성, 위협 및 관리 Shemelyn M. Sespeñe†・Marla Maniquiz-Redillas・김이형・추연욱 Department of Civil and Environmental Engineering, Kongju National University Cheonan City, Korea (Received : 22 June 2016, Revised: 02 August 2016, Accepted: 02 August 2016) Abstract The Philippines is a naturally water-rich archipelago capable of sustaining its ecological goods and providing services and needs of its people. Several waterbodies have been declared as natural wetlands in the country supporting the needs of community like water and food. In this study, 65 natural wetlands were considered including six sites that were identified as ‘Wetlands of International Importance’ such as Naujan Lake National Park, Agusan Marsh Wildlife Sanctuary, Olango Island Wildlife Sanctuary, Tubbataha Reefs Natural Park, Las Piñas-Parañaque Critical Habitat and Ecotourism Area and Puerto Princesa Subterranean River National Park. There are 22 wetland types presented in this research categorizing the Philippine wetlands. Philippine wetlands are now facing tremendous challenges such as land use conversion, abuse of resources, pollution coming from domestic, industrial and agricultural activities, and climate change. This paper provides an overview of Philippine wetlands in terms of their characteristics and components, impacts in the ecosystem, and the challenges they are dealing with. -

Ecological Assessments in the B+WISER Sites
Ecological Assessments in the B+WISER Sites (Northern Sierra Madre Natural Park, Upper Marikina-Kaliwa Forest Reserve, Bago River Watershed and Forest Reserve, Naujan Lake National Park and Subwatersheds, Mt. Kitanglad Range Natural Park and Mt. Apo Natural Park) Philippines Biodiversity & Watersheds Improved for Stronger Economy & Ecosystem Resilience (B+WISER) 23 March 2015 This publication was produced for review by the United States Agency for International Development. It was prepared by Chemonics International Inc. The Biodiversity and Watersheds Improved for Stronger Economy and Ecosystem Resilience Program is funded by the USAID, Contract No. AID-492-C-13-00002 and implemented by Chemonics International in association with: Fauna and Flora International (FFI) Haribon Foundation World Agroforestry Center (ICRAF) The author’s views expressed in this publication do not necessarily reflect the views of the United States Agency for International Development or the United States Government. Ecological Assessments in the B+WISER Sites Philippines Biodiversity and Watersheds Improved for Stronger Economy and Ecosystem Resilience (B+WISER) Program Implemented with: Department of Environment and Natural Resources Other National Government Agencies Local Government Units and Agencies Supported by: United States Agency for International Development Contract No.: AID-492-C-13-00002 Managed by: Chemonics International Inc. in partnership with Fauna and Flora International (FFI) Haribon Foundation World Agroforestry Center (ICRAF) 23 March -
![Use of Field Recorded Sounds in the Assessment of Forest Birds in Palawan, Philippines ______Required in the Field [19]](https://docslib.b-cdn.net/cover/0725/use-of-field-recorded-sounds-in-the-assessment-of-forest-birds-in-palawan-philippines-required-in-the-field-19-570725.webp)
Use of Field Recorded Sounds in the Assessment of Forest Birds in Palawan, Philippines ______Required in the Field [19]
Asia Pacific Journal of Multidisciplinary Research, Vol. 7, No. 2, May, 2019 _____________________________________________________________________________________________________________________ Asia Pacific Journal of Use of Field Recorded Sounds in the Multidisciplinary Research Assessment of Forest Birds in Palawan, Vol. 7 No.2, 24-31 May 2019 Philippines P-ISSN 2350-7756 E-ISSN 2350-8442 Alejandro A. Bernardo Jr. www.apjmr.com College of Arts and Sciences, Western Philippines University, CHED Recognized Journal Aborlan, Palawan, Philippines ASEAN Citation Index [email protected] Date Received: August 3, 2018; Date Revised: February 8, 2019 Abstract -The uses of bioacoustics in biological applications are getting popular in research communities. Among such application is the use of sound recordings in avifaunal researches. This research explored the possibility of using the sound recording in the assessment of forest birds in Palawan by comparing it in widely used Point Count Method (PCM). To compare the two methods, a simultaneous point count and sound recording surveys from February to November 2017 in the forested slopes of Victoria-Anipahan Mountain in Aborlan, Palawan were conducted. The Sound Recording Method (SRM) listed slightly lower species richness than the PCM, but the difference in the mean number of species was not significant (F1,49=1.05, p > 0.05). The SRM was found to be biased towards noisy and loud calling bird species but it failed to detect the silent and rarely calling species. SRM was also equally sensitive as compared to PCM in detecting endemic and high conservation priority species. Because of these, it was recognized that SRM could be used as one of the alternative methods in forest bird assessment particularly if the concern is avifaunal species richness. -

Report-VIC-Croajingolong National Park-Appendix A
Croajingolong National Park, Victoria, 2016 Appendix A: Fauna species lists Family Species Common name Mammals Acrobatidae Acrobates pygmaeus Feathertail Glider Balaenopteriae Megaptera novaeangliae # ~ Humpback Whale Burramyidae Cercartetus nanus ~ Eastern Pygmy Possum Canidae Vulpes vulpes ^ Fox Cervidae Cervus unicolor ^ Sambar Deer Dasyuridae Antechinus agilis Agile Antechinus Dasyuridae Antechinus mimetes Dusky Antechinus Dasyuridae Sminthopsis leucopus White-footed Dunnart Felidae Felis catus ^ Cat Leporidae Oryctolagus cuniculus ^ Rabbit Macropodidae Macropus giganteus Eastern Grey Kangaroo Macropodidae Macropus rufogriseus Red Necked Wallaby Macropodidae Wallabia bicolor Swamp Wallaby Miniopteridae Miniopterus schreibersii oceanensis ~ Eastern Bent-wing Bat Muridae Hydromys chrysogaster Water Rat Muridae Mus musculus ^ House Mouse Muridae Rattus fuscipes Bush Rat Muridae Rattus lutreolus Swamp Rat Otariidae Arctocephalus pusillus doriferus ~ Australian Fur-seal Otariidae Arctocephalus forsteri ~ New Zealand Fur Seal Peramelidae Isoodon obesulus Southern Brown Bandicoot Peramelidae Perameles nasuta Long-nosed Bandicoot Petauridae Petaurus australis Yellow Bellied Glider Petauridae Petaurus breviceps Sugar Glider Phalangeridae Trichosurus cunninghami Mountain Brushtail Possum Phalangeridae Trichosurus vulpecula Common Brushtail Possum Phascolarctidae Phascolarctos cinereus Koala Potoroidae Potorous sp. # ~ Long-nosed or Long-footed Potoroo Pseudocheiridae Petauroides volans Greater Glider Pseudocheiridae Pseudocheirus peregrinus -

Lepidoptera: Tortricidae: Tortricinae) and Evolutionary Correlates of Novel Secondary Sexual Structures
Zootaxa 3729 (1): 001–062 ISSN 1175-5326 (print edition) www.mapress.com/zootaxa/ Monograph ZOOTAXA Copyright © 2013 Magnolia Press ISSN 1175-5334 (online edition) http://dx.doi.org/10.11646/zootaxa.3729.1.1 http://zoobank.org/urn:lsid:zoobank.org:pub:CA0C1355-FF3E-4C67-8F48-544B2166AF2A ZOOTAXA 3729 Phylogeny of the tribe Archipini (Lepidoptera: Tortricidae: Tortricinae) and evolutionary correlates of novel secondary sexual structures JASON J. DOMBROSKIE1,2,3 & FELIX A. H. SPERLING2 1Cornell University, Comstock Hall, Department of Entomology, Ithaca, NY, USA, 14853-2601. E-mail: [email protected] 2Department of Biological Sciences, University of Alberta, Edmonton, Canada, T6G 2E9 3Corresponding author Magnolia Press Auckland, New Zealand Accepted by J. Brown: 2 Sept. 2013; published: 25 Oct. 2013 Licensed under a Creative Commons Attribution License http://creativecommons.org/licenses/by/3.0 JASON J. DOMBROSKIE & FELIX A. H. SPERLING Phylogeny of the tribe Archipini (Lepidoptera: Tortricidae: Tortricinae) and evolutionary correlates of novel secondary sexual structures (Zootaxa 3729) 62 pp.; 30 cm. 25 Oct. 2013 ISBN 978-1-77557-288-6 (paperback) ISBN 978-1-77557-289-3 (Online edition) FIRST PUBLISHED IN 2013 BY Magnolia Press P.O. Box 41-383 Auckland 1346 New Zealand e-mail: [email protected] http://www.mapress.com/zootaxa/ © 2013 Magnolia Press 2 · Zootaxa 3729 (1) © 2013 Magnolia Press DOMBROSKIE & SPERLING Table of contents Abstract . 3 Material and methods . 6 Results . 18 Discussion . 23 Conclusions . 33 Acknowledgements . 33 Literature cited . 34 APPENDIX 1. 38 APPENDIX 2. 44 Additional References for Appendices 1 & 2 . 49 APPENDIX 3. 51 APPENDIX 4. 52 APPENDIX 5. -
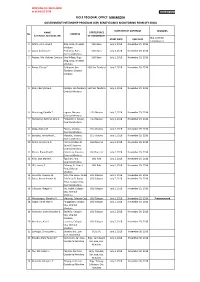
Dole Regional Office Mimaropa Government Internship Program (Gip) Beneficiaries Monitoring Form (Fy 2018)
PROFILING OF CHILD LABOR as of July 25, 2018 DOLE-GIP_Form C DOLE REGIONAL OFFICE MIMAROPA GOVERNMENT INTERNSHIP PROGRAM (GIP) BENEFICIARIES MONITORING FORM (FY 2018) DURATION OF CONTRACT REMARKS NAME OFFICE/PLACE No. ADDRESS (Last Name, First Name, MI) OF ASSIGNMENT (e.g. Contract START DATE END DATE completed or 1 Alforo, John Lloyd Z. Alag, Baco, Oriental LGU Baco July 2, 2018 November 29, 2018 Mindoro 2 Lapat, Anthony O. Poblacion, Baco, LGU Baco July 2, 2018 November 29, 2018 Oriental Mindoro 3 Nebres, Ma. Dolores Corazon A.Sitio Hilltop, Brgy. LGU Baco July 2, 2018 November 29, 2018 Alag, Baco, Oriental Mindoro 4 Rance, Elaesa E. Poblacion, San LGU San Teodoro July 2, 2018 November 29, 2018 Teodoro, Oriental Mindoro 5 Rizo, CherryMae A. Calsapa, San Teodoro, LGU San Teodoro July 2, 2018 November 29, 2018 Oriental Mindoro 6 Macarang, Cybelle T. Laguna, Naujan, LGU Naujan July 2, 2018 November 29, 2018 Oriental Mindoro 7 Mantaring, Kathryn Jane A. Poblacion II, Naujan, LGU Naujan July 2, 2018 November 29, 2018 Oriental Mindoro 8 Abog, Orpha M. Pakyas, Victoria, LGU Victoria July 2, 2018 November 29, 2018 Oriental Mindoro 9 Boncato, Jenna Mae C. Macatoc, Victoria, LGU Victoria July 2, 2018 November 29, 2018 Oriental Mindoro 10 Nefiel, Jeric John D. Flores de Mayo St. LGU Socorro July 2, 2018 November 29, 2018 Zone IV, Socorro, Oriental Mindoro 11 Platon, Bryan Paul R. Calocmoy, Socorro, LGU Socorro July 2, 2018 November 29, 2018 Oriental Mindoro 12 Nillo, Joza Marie D. Tiguihan, Pola, LGU Pola July 2, 2018 November 29, 2018 Oriental Mindoro 13 Ulit, Lovely E. -

PSA Oriental Mindoro Conducts Enumerators Training And
Volume 1, Issue 4 JULY - SEPTEMBER 2019 IN THIS ISSUE PSA Oriental Mindoro conducts Enumerators Training and Workshop in MinSCAT Main Campus PSA Oriental Mindoro conducts Enumerators Training and PSA Oriental Mindoro conducted Workshop in MinSCAT Main enumerators training and workshop Campus in Mindoro State College of Agricul- ture and Technology Main Campus PSA Oriental Mindoro joins Victoria on August 22, 28 and 29, Takbo Para sa Kabataan: Bubble 2019. The three day training was Run 2019 and The 404th conducted to prepare Statistical Researchers hired by the academe Maneuver Company: Run for in the Assessment Survey on the Mindoro Needs and Opportunities for Future Participants and PSA OrMin Personnel Partnership with Local Farmers and involved in the training. PSA Oriental Mindoro Industry. conducts Information Dissemination in Schools The training focused on the discussion of Interview Techniques, Role of Statistical Researchers, Data Presentation, Data Processing, and Survey Operations & Questionnaire Design. Thirty (30) Statistical Researchers, PSA OrMin Attendance for two from each municipality of the province, were involved while CSS Learning Efren C. Armonia, SSS Charlyn Romero-Cantos and SS II Herchie T. Davalos of PSA Oriental Mindoro facilitated the training. PSA Oriental Mindoro joins Takbo Para sa Kabataan: Bubble Run 2019 PSA OrMin Attendance for and The 404th Maneuver Company: Run for Mindoro Partnership CPI & Inflation Rate in the Province Did You Know That The 404th Maneuver Company: Run Takbo Para sa Kabataan: Bubble for Mindoro Run 2019 PSA Oriental Mindoro joined two fun run activities in Calapan City. On August 10, 2019, SK Federation organized Takbo Para sa Kabataan: Bubble Run 2019 as part of “Linggo ng Kabataan” celebration while on September 8, 2019 the AgriStat Corner 404th Maneuver Company: Run for Mindoro was initiated.