An Overview of Immune Hemolytic Anemias
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Hemolysis and Venous Thrombosis: Which Link? A
ISSN: 2378-3656 Rkiouak et al. Clin Med Rev Case Rep 2020, 7:329 DOI: 10.23937/2378-3656/1410329 Volume 7 | Issue 11 Clinical Medical Reviews Open Access and Case Reports ORIGINAL RESEARCH Hemolysis and Venous Thrombosis: Which Link? A. Rkiouak, PH.D1, I El Kassimi, MD2, N Sahel, MD2, M Zaizaa, MD2 and Y Sekkach, PhD1 Check for updates Internal Medicine A Department, Mohammed V Military Hospital Medical School of Rabat, Morocco *Corresponding author: Adil Rkiouak, PH.D., Department of Internal Medicine A, Mohammed V Military Hospital, Medical School of Rabat, Morocco, Tel: +212-66-179-44-04 The mechanism of antibody-mediated hemolysis is Abstract via phagocytosis or complement-mediated destruction The association hemolysis and venous thrombosis remains and can occur intravascular or extravascular. The intra- unknown to clinicians, despite our advances in comrehen- sion of pathophysiological bases. vascular mechanisms include direct cellular destruction via lysis, toxins, or trauma; fragmentation and oxida- Haemolysis, which is observed in multiple diseases, can affect all three components of Virchow’s triad. It is not sur- tion. prising that there is a link between haemolytic disorders and Multiple haemolytic disorders produce substantial thrombosis. intravascular haemolysis. Examples, the corpuscular We will try to clarify the main pro-thrombotic mechanisms hemolysis include PNH, extra-corpuscular haemolysis, during hemolysis through 3 clinical observations of deep ve- acquired (autoimmune haemolytic anaemia (AIHA , nous thrombosis in 3 main types of hemolytic pathologies, ) namely a case of paroxysmal nocturnal hemoglobinuria, thrombotic thrombocytopenic purpura (PTT)), as well thrombotic thrombocytopenic purpura and autoimmune as other diseases. These disorders are also associated anemia hemolytic. -

Clostridial Septicemia with Intravascular Hemolysis: a Case Report G
Henry Ford Hospital Medical Journal Volume 13 | Number 4 Article 4 12-1965 Clostridial Septicemia With Intravascular Hemolysis: A Case Report G. M. Mastio E. Morfin Follow this and additional works at: https://scholarlycommons.henryford.com/hfhmedjournal Part of the Life Sciences Commons, Medical Specialties Commons, and the Public Health Commons Recommended Citation Mastio, G. M. and Morfin, E. (1965) "Clostridial Septicemia With Intravascular Hemolysis: A Case Report," Henry Ford Hospital Medical Bulletin : Vol. 13 : No. 4 , 421-425. Available at: https://scholarlycommons.henryford.com/hfhmedjournal/vol13/iss4/4 This Article is brought to you for free and open access by Henry Ford Health System Scholarly Commons. It has been accepted for inclusion in Henry Ford Hospital Medical Journal by an authorized editor of Henry Ford Health System Scholarly Commons. Henry Ford Hosp. Med. Bull. Vol. 13, December 1965 CLOSTRIDIAL SEPTICEMIA WITH INTRAVASCULAR HEMOLYSIS A CASE REPORT G. M. MASTIC, M.D. AND E. MORFIN, M.D. In 1871 Bottini' demonstrated the bacterial nature of gas gangrene, but failed to isolate a causal organism. Clostridium perfringens, sometimes known as Clostridium welchii, was discovered independently during 1892 and 1893 by Welch, Frankel, "Veillon and Zuber.^ This organism is a saprophytic inhabitant of the intestinal tract, and may be a harmless saprophyte of the female genital tract occurring in the vagina in 4-6 per cent of pregnant women. Clostridial organisms occur in great numbers and distribution throughout the world. Because of this, they are very common in traumatic wounds. Very few species of Clostridia, however, are pathogenic, and still fewer are capable of producing gas gangrene in man. -
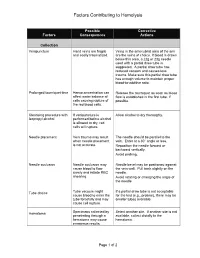
Factors Contributing to Hemolysis
Factors Contributing to Hemolysis Possible Corrective Factors Consequences Actions Collection Venipuncture Hand veins are fragile Veins in the antecubital area of the arm and easily traumatized. are the veins of choice. If blood is drawn below this area, a 22g or 23g needle used with a partial draw tube is suggested. A partial draw tube has reduced vacuum and causes less trauma. Make sure this partial draw tube has enough volume to maintain proper blood-to-additive ratio. Prolonged tourniquet time Hemoconcentration can Release the tourniquet as soon as blood affect water balance of flow is established in the first tube, if cells causing rupture of possible. the red blood cells. Cleansing procedure with If venipuncture is Allow alcohol to dry thoroughly. isopropyl alcohol performed before alcohol is allowed to dry, red cells will rupture. Needle placement Vein trauma may result The needle should be parallel to the when needle placement vein. Enter at a 30° angle or less. is not accurate. Reposition the needle forward or backward vertically. Avoid probing. Needle occlusion Needle occlusion may Needle bevel may be positioned against cause blood to flow the vein wall. Pull back slightly on the slowly and initiate RBC needle. shearing. Avoid rotating or changing the angle of the needle. Tube choice Tube vacuum might If a partial draw tube is not acceptable cause blood to enter the for the test (e.g., protime), there may be tube forcefully and may smaller tubes available. cause cell rupture. Hematoma Specimens collected by Select another site. If another site is not penetrating through a available, collect distally to the hematoma may cause hematoma. -

Human Plasma and Recombinant Hemopexins: Heme Binding Revisited
International Journal of Molecular Sciences Article Human Plasma and Recombinant Hemopexins: Heme Binding Revisited Elena Karnaukhova 1,*, Catherine Owczarek 2, Peter Schmidt 2, Dominik J. Schaer 3 and Paul W. Buehler 4,5,* 1 Center for Biologics Evaluation and Research, Food and Drug Administration, Silver Spring, MD 20993, USA 2 CSL Limited, Bio21 Institute, Parkville, Victoria 3010, Australia; [email protected] (C.O.); [email protected] (P.S.) 3 Division of Internal Medicine, University Hospital of Zurich, 8091 Zurich, Switzerland; [email protected] 4 Department of Pathology, The University of Maryland School of Medicine, Baltimore, MD 21201, USA 5 The Center for Blood Oxygen Transport and Hemostasis, Department of Pediatrics, The University of Maryland School of Medicine, Baltimore, MD 21201, USA * Correspondence: [email protected] (E.K.); [email protected] (P.W.B.) Abstract: Plasma hemopexin (HPX) is the key antioxidant protein of the endogenous clearance pathway that limits the deleterious effects of heme released from hemoglobin and myoglobin (the term “heme” is used in this article to denote both the ferrous and ferric forms). During intra-vascular hemolysis, heme partitioning to protein and lipid increases as the plasma concentration of HPX declines. Therefore, the development of HPX as a replacement therapy during high heme stress could be a relevant intervention for hemolytic disorders. A logical approach to enhance HPX yield involves recombinant production strategies from human cell lines. The present study focuses on a biophysical assessment of heme binding to recombinant human HPX (rhHPX) produced in the Expi293FTM (HEK293) cell system. -

A Suspected Case of Clostridium Perfringens Sepsis With
Uojima et al. Journal of Medical Case Reports (2019) 13:125 https://doi.org/10.1186/s13256-019-2023-x CASE REPORT Open Access A suspected case of Clostridium perfringens sepsis with intravascular hemolysis after transhepatic arterial chemoembolization: a case report Haruki Uojima1,2*, Mie Onoue2, Hisashi Hidaka2, Naohisa Wada2, Yoshiaki Tanaka2, Tomoyoshi Inoue2, Kousuke Kubota2, Takahide Nakazawa2, Akitaka Shibuya2 and Wasaburo Koizumi2 Abstract Introduction: Sepsis due to Clostridium perfringens, one of several clostridial species, is an important cause of massive intravascular hemolysis in patients with underlying malignancies. Chronic liver diseases, immunosuppression, and presence of malignancies were risk factors for Clostridium perfringens sepsis. Therefore, Clostridium perfringens sepsis should always be considered in patients presenting with liver damage after chemo- embolic therapy for hepatocellular carcinoma. This case report focuses on findings characteristic of an intravascular hemolysis due to Clostridium perfringens after transhepatic arterial chemoembolization. Case presentation: An 83-year-old Japanese man presented to our hospital because of a third recurrence of hepatocellular carcinoma. He had nonalcoholic steatohepatitis-related cirrhosis, and underwent radiofrequency ablation and transhepatic arterial chemoembolization therapy for hepatocellular carcinoma of S4/S8 and S2. He had a medical history of pancreatic carcinoma and underwent pylorus-preserving pancreaticoduodenectomy approximately 5 years ago. Because follow-up -

MASSHEALTH TRANSMITTAL LETTER LAB-22 July 2002 TO
Commonwealth of Massachusetts Executive Office of Health and Human Services Division of Medical Assistance 600 Washington Street Boston, MA 02111 www.mass.gov/dma MASSHEALTH TRANSMITTAL LETTER LAB-22 July 2002 TO: Independent Clinical Laboratories Participating in MassHealth FROM: Wendy E. Warring, Commissioner RE: Independent Clinical Laboratory Manual (Laboratory HCPCS) The federal government has revised the HCFA Common Procedure Coding System (HCPCS) for MassHealth billing. This letter transmits changes for your provider manual that contain the new and revised codes. The revised Subchapter 6 is effective for dates of service on or after April 30, 2002. The codes introduced under the 2002 HCPCS code book are effective for dates of service on or after April 30, 2002. We will accept either the new or the old codes for dates of service through July 28, 2002. For dates of service on or after July 29, 2002, you must use the new codes to receive payment. If you wish to obtain a fee schedule, you may purchase Division of Health Care Finance and Policy regulations from either the Massachusetts State Bookstore or from the Division of Health Care Finance and Policy (see addresses and telephone numbers below). You must contact them first to find out the price of the publication. The Division of Health Care Finance and Policy also has the regulations available on disk. The regulation title for laboratory is 114.3 CMR 20.00: Laboratory. Massachusetts State Bookstore Division of Health Care Finance and Policy State House, Room 116 Two Boylston Street -

Clotting Phenomena at the Blood-Polymer Interface and Development of Blood Compatible Polymeric Surfaces
Clotting Phenomena at the Blood-Polymer Interface and Development of Blood Compatible Polymeric Surfaces Adriaan Bantjes In the past two decades many attempts have been made to relate surface and interfacial parameters with the blood compatibility of polymeric surfaces. It is however doubtful if by a single parameter the behaviour of blood on a surface can be predicted. Two major aspects of blood compatibility -- the prevention of platelet adhesion and the deactivation of the intrinsic coagulation system are determined by the measure and nature of competi- tive blood protein adsorption on the foreign surface. The adhesion of blood platelets is promoted by adsorbed fibrinogen and gamma globulin, while adsorbed albumin inhibits platelct adhesion. Heparinised surfaces do not adsorb fibrin and consequently no adhesion of platelets takes place. Othcr surfaces with low platelct adhesion are the hydrogels, certain block copolyetherurethanes, polyelectrolyte complexes and biolised proteins. Heparinised surfaces of the cationically bonded type inhibit the intrinsic coagulation as well, however this may be due to unstable coatings and heparin leakage. In the authors laboratory a synthetic heparinoid was prepared with the structure - [CH, - C(CH3) NHS03Na ~ C(H) COONa ~ CH2 --] with h, = (7.5 5 1 .O) x lo5 and an in vivo anticoagulant activity of 50% of heparin. Its coatings on PVC, using tridodecyltiietliyl-ammoniuni chloridc as a coupling agent, are stable in plasma and salt solutions and provide surfaces which show negligible platelet adhesion and a strong inhibition 01 the intrinsic coagulation on contact with blood. Similar results were found with polydimethylsiloxane surfaces coated with this heparinoid. 1. INTRODUCTION new blood compatible materials may be developed it is necessary to explain sotne of the mechanisms of blood co- The clinical use of blood contacting devices and prosthcses is agulation on intcraction with a foreign surface. -

20 Hemolytic Anemias Due to Abnormal Red Cell Enzymes
Hemolytic Anemias Due to Abnormal Red Cell Enzymes MODULE Hematology and Blood Bank Technique 20 HEMOLYTIC ANEMIAS DUE TO Notes ABNORMAL RED CELL ENZYMES 20.1 INTRODUCTION The main metabolic substrate for the RBCs is glucose. It is metabolized by two pathways: approximately 90% of the glucose is metabolized through the Embden Meyerhoff (glycolytic) pathway and the rest by the hexose monophosphate (HMP) pathway. In the Embden Meyerhoff (glycolytic) pathway glucose is metabolized to lactate through a series of enzymatic steps. Each molecule of glucose gives rise to 2 molecules of ATP. The ATP provides energy to maintain red cell volume, shape and flexibility. An ATP dependent pump in the red cell membrane actively keeps sodium out of the cell and potassium inside. The red cell has the enzymes that are needed for the glycolytic pathway. These enzymes help break down glucose to generate ATP which is the source of energy. About 10% of the glucose is diverted to the Hexose Monophosphate shunt pathway and this is essential for protection of red cells from oxidative stress. This pathway is necessary for the generation of NADPH which then reduces oxidized glutathione (GSSG) to reduced glutathione (GSH). GSH prevents the accumulation of H2O2 and the oxidation of hemoglobin to methemoglobin. When the level of GSH falls, H2O2 accumulates in the cell and oxidizes the hemoglobin to methemoglobin which becomes denatured and precipitates as Heinz bodies. These inclusions are rigid and attached to the red cell membrane and make the red cell susceptible to hemolysis. The NADPH required in this pathway is generated by the enzyme Glucose 6 phosphate dehydrogenase (G6PD). -

The Effect of Gamma Globulin in Pustular Acne*
PRELIMINARY AND SHORT REPORT THE EFFECT OF GAMMA GLOBULIN IN PUSTULAR ACNE* LOREN W.SHAFFER,M.D., BENJAMIN SdHWIMMER, M.D. AND RENATO J.STANICCO,M.D. One of us (LWS) bad occasion to treat a young Clinical Response: Patient 1: New lesions ceased white man with gamma globulin for prophylaxisappearing immediately after the first injection. after exposure to infectious hepatitis. He wasBy the third week most of the cystic and pustular given two injections, 10 cc. each, of human Polio-lesions had dried up, and no new ones had ap- myelitis Immune Globulin, one week apart. Itpeared. Comedones were not decreased in number. was observed that shortly after the second injec-The patient maintained his improvement through tion, his severe pustular and indurated acnethe fifth week when the cystic and pustular lesions improved considerably. This observation led tobegan to recur. the present study, of which this is a preliminary Patient 2: New lesions continued to develop report. throughout the period of treatment and the one This study of the therapeutic effect of gammamonth follow up. globulin in pustular acne is coupled with a study Patient 3: There was no observable effect of of the serum protein and C-reactive protein levelstreatment. before and after treatment. Patient 4: The first improvement was seen in Method: Four patients in their late teens, other-the second week and by the third, all cystic and wise healthy, but with severe pustular and cysticpustular lesions were dry. The improvement acne that had been resistant to conventionallasted for two weeks after which the lesions methods of treatment were selected for the study.began to return. -

Syllabus: Page 23
The University of Texas at El Paso College of Health Sciences Clinical Laboratory Science Program CLSC 3364 Hematology II Course Outline Spring What do you see? What is in your Head? Video or audio recordings will not be permitted. Instructor M. Lorraine Torres, Ed. D, MT (ASCP) College of Health Sciences Room 423 Phone: 747-7282 E-Mail: [email protected] Office Hours TR 3:00 – 4:00 p.m., Friday 2 – 3 p.m. or by appointment Class Schedule Monday and Wednesday 11:00 – 12:30 A.M. HSCI 135 Course Description This course is a sequel to Hematology I. It will include but is not limited to the study of the white blood cells with emphasis on white cell formation and function and the etiology and treatment of white blood cell disorders. This course will also encompass an introduction to hemostasis and laboratory determination of hemostatic disorders. Prerequisite; CLSC 3356 & CLSC 3257. Topical Outline 1. Maturation series and biology of white blood cells 2. Disorders of neutrophils 3. Reactive lymphocytes and Infectious Mononucleosis 4. Acute and chronic leukemias 5. Myelodysplastic syndromes 6. Myeloproliferative disorders 7. Multiple Myeloma and related plasma cell disorders 8. Lymphomas 9. Lipid (lysosomal) storage diseased and histiosytosis 10. Hemostatic mechanisms, platelet biology 11. Coagulation pathways 12. Quantitative and qualitative vascular and platelet disorders (congenital and acquired) 13. Disorders of plasma clotting factors 14. Interaction of the fibrinolytic, coagulation and kinin systems 15. Laboratory methods REQUIRED TEXTBOOKS: same books used for Hematology I Keohane, E.M., Smith, L.J. and Walenga, J.M. 2016. Rodak’s Hematology: Clinical Principles and applications. -

Pathological Conditions Involving Extracellular Hemoglobin
Pathological Conditions Involving Extracellular Hemoglobin: Molecular Mechanisms, Clinical Significance, and Novel Therapeutic Opportunities for alpha(1)-Microglobulin Gram, Magnus; Allhorn, Maria; Bülow, Leif; Hansson, Stefan; Ley, David; Olsson, Martin L; Schmidtchen, Artur; Åkerström, Bo Published in: Antioxidants & Redox Signaling DOI: 10.1089/ars.2011.4282 2012 Link to publication Citation for published version (APA): Gram, M., Allhorn, M., Bülow, L., Hansson, S., Ley, D., Olsson, M. L., Schmidtchen, A., & Åkerström, B. (2012). Pathological Conditions Involving Extracellular Hemoglobin: Molecular Mechanisms, Clinical Significance, and Novel Therapeutic Opportunities for alpha(1)-Microglobulin. Antioxidants & Redox Signaling, 17(5), 813-846. https://doi.org/10.1089/ars.2011.4282 Total number of authors: 8 General rights Unless other specific re-use rights are stated the following general rights apply: Copyright and moral rights for the publications made accessible in the public portal are retained by the authors and/or other copyright owners and it is a condition of accessing publications that users recognise and abide by the legal requirements associated with these rights. • Users may download and print one copy of any publication from the public portal for the purpose of private study or research. • You may not further distribute the material or use it for any profit-making activity or commercial gain • You may freely distribute the URL identifying the publication in the public portal Read more about Creative commons licenses: https://creativecommons.org/licenses/ Take down policy If you believe that this document breaches copyright please contact us providing details, and we will remove access to the work immediately and investigate your claim. -

SEED Haematology Sysmex Educational Enhancement and Development October 2012
SEED Haematology Sysmex Educational Enhancement and Development October 2012 The red blood cell indices The full blood count has been used in conjunction with the traditional red The complete blood count (CBC) is central to clinical deci- cell indices in order to narrow down the possible causes sion making. This makes it one of the commonest laboratory of anaemia in an individual patient. investigations performed worldwide. Whilst the definition of what constitutes an CBC is influenced by the number Impedance technology and type of parameters measured by different haematology The RBC, HCT and MCV are all closely interrelated as they analysers, the traditional red cell indices that are widely are derived from information obtained from the passage used to classify anaemias are common to all. of cells through the aperture of the impedance channel of an automated haematology analyser. The impedance The laboratory approach to anaemia technology is based on the principle that an electrical field, Anaemia is an extremely common global healthcare prob- created between two electrodes of opposite charge, can lem. However, anaemia is merely a symptom which can be used to count and determine the size of cells. Blood result from a multitude of causes. Effective treatment is cells are poor conductors of electricity. The diluent in which only possible if the underlying cause is correctly identified. they are suspended as they pass through the aperture To this end, several classification systems have been devis- during counting is an isotonic solution which is a good ed. The most useful and widely used classification system conductor of electricity.