Latham & Ricklefs 1993.Indd
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Field Release of the Leaf-Feeding Moth, Hypena Opulenta (Christoph)
United States Department of Field release of the leaf-feeding Agriculture moth, Hypena opulenta Marketing and Regulatory (Christoph) (Lepidoptera: Programs Noctuidae), for classical Animal and Plant Health Inspection biological control of swallow- Service worts, Vincetoxicum nigrum (L.) Moench and V. rossicum (Kleopow) Barbarich (Gentianales: Apocynaceae), in the contiguous United States. Final Environmental Assessment, August 2017 Field release of the leaf-feeding moth, Hypena opulenta (Christoph) (Lepidoptera: Noctuidae), for classical biological control of swallow-worts, Vincetoxicum nigrum (L.) Moench and V. rossicum (Kleopow) Barbarich (Gentianales: Apocynaceae), in the contiguous United States. Final Environmental Assessment, August 2017 Agency Contact: Colin D. Stewart, Assistant Director Pests, Pathogens, and Biocontrol Permits Plant Protection and Quarantine Animal and Plant Health Inspection Service U.S. Department of Agriculture 4700 River Rd., Unit 133 Riverdale, MD 20737 Non-Discrimination Policy The U.S. Department of Agriculture (USDA) prohibits discrimination against its customers, employees, and applicants for employment on the bases of race, color, national origin, age, disability, sex, gender identity, religion, reprisal, and where applicable, political beliefs, marital status, familial or parental status, sexual orientation, or all or part of an individual's income is derived from any public assistance program, or protected genetic information in employment or in any program or activity conducted or funded by the Department. (Not all prohibited bases will apply to all programs and/or employment activities.) To File an Employment Complaint If you wish to file an employment complaint, you must contact your agency's EEO Counselor (PDF) within 45 days of the date of the alleged discriminatory act, event, or in the case of a personnel action. -

Endangered Species (Protection, Conser Va Tion and Regulation of Trade)
ENDANGERED SPECIES (PROTECTION, CONSER VA TION AND REGULATION OF TRADE) THE ENDANGERED SPECIES (PROTECTION, CONSERVATION AND REGULATION OF TRADE) ACT ARRANGEMENT OF SECTIONS Preliminary Short title. Interpretation. Objects of Act. Saving of other laws. Exemptions, etc., relating to trade. Amendment of Schedules. Approved management programmes. Approval of scientific institution. Inter-scientific institution transfer. Breeding in captivity. Artificial propagation. Export of personal or household effects. PART I. Administration Designahem of Mana~mentand establishment of Scientific Authority. Policy directions. Functions of Management Authority. Functions of Scientific Authority. Scientific reports. PART II. Restriction on wade in endangered species 18. Restriction on trade in endangered species. 2 ENDANGERED SPECIES (PROTECTION, CONSERVATION AND REGULA TION OF TRADE) Regulation of trade in species spec fled in the First, Second, Third and Fourth Schedules Application to trade in endangered specimen of species specified in First, Second, Third and Fourth Schedule. Export of specimens of species specified in First Schedule. Importation of specimens of species specified in First Schedule. Re-export of specimens of species specified in First Schedule. Introduction from the sea certificate for specimens of species specified in First Schedule. Export of specimens of species specified in Second Schedule. Import of specimens of species specified in Second Schedule. Re-export of specimens of species specified in Second Schedule. Introduction from the sea of specimens of species specified in Second Schedule. Export of specimens of species specified in Third Schedule. Import of specimens of species specified in Third Schedule. Re-export of specimens of species specified in Third Schedule. Export of specimens specified in Fourth Schedule. PART 111. -

Long-Term Trends in Food Habits of the Raccoon Dog, Nyctereutes Viverrinus, in the Imperial Palace, Tokyo
Bull. Natl. Mus. Nat. Sci., Ser. A, 42(3), pp. 143–161, August 22, 2016 Long-term Trends in Food Habits of the Raccoon Dog, Nyctereutes viverrinus, in the Imperial Palace, Tokyo Akihito1, Takako Sako2, Makito Teduka3 and Shin-ichiro Kawada4* 1The Imperial Residence, 1–1 Chiyoda, Chiyoda-ku, Tokyo 100–0001, Japan 2Imperial Household Agency, 1–1 Chiyoda, Chiyoda-ku, Tokyo 100–8111, Japan 3Field Work Office, 4–29–2 Asahi-cho, Akishima, Tokyo 196–0025, Japan 4 Department of Zoology, National Museum of Nature and Science, 4–1–1 Amakubo, Tsukuba, Ibaraki 305–0005, Japan *E-mail: [email protected] (Received 31 March 2016; accepted 22 June 2016) Abstract The food habits of the raccoon dogs in the Imperial Palace were examined by fecal analysis focused on the long term trend for five years. A total of 95 taxa (including 58 taxa identi- fied as genera or species) of plant seeds were detected from 163 collected feces in 164 weekly sur- veys. Among them, eight taxa were selected as the food resources for the raccoon dogs in the Imperial Palace. The intakes of these taxa showed seasonal succession, i.e. Aphananthe aspera in January, Idesia polycarpa in February, Rubus hirsutus from May to July, Cerasus spp. in May and June, Morus spp. in June, Machilus thunbergii in July and August, Aphananthe aspera from Sep- tember to December until the following January, and also Ficus erecta in September and Celtis sinensis in December. In March and April, plant harvest is rather poor, and therefore raccoon dogs feed on the inside endosperm of Ginkgo biloba and family Fagaceae to supply the insufficient nutrients as observed by broken seed coats from feces. -

Reporton the Rare Plants of Puerto Rico
REPORTON THE RARE PLANTS OF PUERTO RICO tii:>. CENTER FOR PLANT CONSERVATION ~ Missouri Botanical Garden St. Louis, Missouri July 15, l' 992 ACKNOWLEDGMENTS The Center for Plant Conservation would like to acknowledge the John D. and Catherine T. MacArthur Foundation and the W. Alton Jones Foundation for their generous support of the Center's work in the priority region of Puerto Rico. We would also like to thank all the participants in the task force meetings, without whose information this report would not be possible. Cover: Zanthoxy7um thomasianum is known from several sites in Puerto Rico and the U.S . Virgin Islands. It is a small shrub (2-3 meters) that grows on the banks of cliffs. Threats to this taxon include development, seed consumption by insects, and road erosion. The seeds are difficult to germinate, but Fairchild Tropical Garden in Miami has plants growing as part of the Center for Plant Conservation's .National Collection of Endangered Plants. (Drawing taken from USFWS 1987 Draft Recovery Plan.) REPORT ON THE RARE PLANTS OF PUERTO RICO TABLE OF CONTENTS Acknowledgements A. Summary 8. All Puerto Rico\Virgin Islands Species of Conservation Concern Explanation of Attached Lists C. Puerto Rico\Virgin Islands [A] and [8] species D. Blank Taxon Questionnaire E. Data Sources for Puerto Rico\Virgin Islands [A] and [B] species F. Pue~to Rico\Virgin Islands Task Force Invitees G. Reviewers of Puerto Rico\Virgin Islands [A] and [8] Species REPORT ON THE RARE PLANTS OF PUERTO RICO SUMMARY The Center for Plant Conservation (Center) has held two meetings of the Puerto Rlco\Virgin Islands Task Force in Puerto Rico. -

Suncoast Grapevine
www.ficuswww.suncoastnps.org.usf.edu/orgs/suncoast The Suncoast Grapevine Newsletter of the Suncoast Native Plant Society, Inc. Volume 36 Number 2 February 2019 FEBRUARY CHAPTER MEETING --- At the Seminole Heights Library --- 4711 Central Ave. Tampa, Florida 33603-3905 Dispersion and Impacts of Texas Phoenix Palm decline on Sabal palmetto at the Golden Aster Preserve Presented by Chris Hanni Wednesday, February 20 at 7 PM Texas Phoenix Palm Decline (TPPD) is a new disease in Florida, first appearing between Tampa and Sarasota in 2006-2008. Hillsborough County has been an epicenter for the disease which has spread to 22 Florida counties and which affects several species of date palm as well as our state tree, the cabbage palm (Sabal palmetto). The disease is fatal and there is presently no cure. About the Speaker: Chris is a graduate student at the school of GeoSciences, University of South Florida. His goal is to increase awareness of the disease and how it’s impacting the Sabal palmetto (our state tree). Chris and his wife Rebekah are combat veterans (6 deployments). They have 2 children and have lived in Hills- borough County since 2007. He has an A.S. in Computer Science, a B.S. in Environmental Microbiology and a Masters in Geography (GIS and Spatial Analysis). He is planning a PhD in Geography and Environmental Science Policy to start this fall. In his spare time he writes music and works on his 1977 CJ5. Light refreshments will not be served at the library, a native plant donation auction follows the presentation. - submitted by Virginia Overstreet Note - The Suncoast Chapter’s Board of Directors has designated February’s regular monthly meeting to elect officers and the 2019 Board of Directors. -

Lyonia Preserve Plant Checklist
Lyonia Preserve Plant Checklist Volusia County, Florida Aceraceae (Maple) Asteraceae (Aster) Red Maple Acer rubrum Bitterweed Helenium amarum Blackroot Pterocaulon virgatum Agavaceae (Yucca) Blazing Star Liatris sp. Adam's Needle Yucca filamentosa Blazing Star Liatris tenuifolia Nolina Nolina brittoniana Camphorweed Heterotheca subaxillaris Spanish Bayonet Yucca aloifolia Cudweed Gnaphalium falcatum Dog Fennel Eupatorium capillifolium Amaranthaceae (Amaranth) Dwarf Horseweed Conyza candensis Cottonweed Froelichia floridana False Dandelion Pyrrhopappus carolinianus Fireweed Erechtites hieracifolia Anacardiaceae (Cashew) Garberia Garberia heterophylla Winged Sumac Rhus copallina Goldenaster Pityopsis graminifolia Goldenrod Solidago chapmanii Annonaceae (Custard Apple) Goldenrod Solidago fistulosa Flag Paw paw Asimina obovata Goldenrod Solidago spp. Mohr's Throughwort Eupatorium mohrii Apiaceae (Celery) Ragweed Ambrosia artemisiifolia Dollarweed Hydrocotyle sp. Saltbush Baccharis halimifolia Spanish Needles Bidens alba Apocynaceae (Dogbane) Wild Lettuce Lactuca graminifolia Periwinkle Catharathus roseus Brassicaceae (Mustard) Aquifoliaceae (Holly) Poorman's Pepper Lepidium virginicum Gallberry Ilex glabra Sand Holly Ilex ambigua Bromeliaceae (Airplant) Scrub Holly Ilex opaca var. arenicola Ball Moss Tillandsia recurvata Spanish Moss Tillandsia usneoides Arecaceae (Palm) Saw Palmetto Serenoa repens Cactaceae (Cactus) Scrub Palmetto Sabal etonia Prickly Pear Opuntia humifusa Asclepiadaceae (Milkweed) Caesalpinceae Butterfly Weed Asclepias -
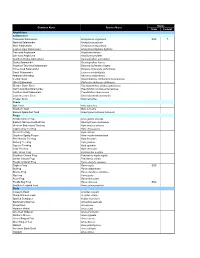
St. Joseph Bay Native Species List
Status Common Name Species Name State Federal Amphibians Salamanders Flatwoods Salamander Ambystoma cingulatum SSC T Marbled Salamander Ambystoma opacum Mole Salamander Ambystoma talpoideum Eastern Tiger Salamander Ambystoma tigrinum tigrinum Two-toed Amphiuma Amphiuma means One-toed Amphiuma Amphiuma pholeter Southern Dusky Salamander Desmognathus auriculatus Dusky Salamander Desmognathus fuscus Southern Two-lined Salamander Eurycea bislineata cirrigera Three-lined Salamander Eurycea longicauda guttolineata Dwarf Salamander Eurycea quadridigitata Alabama Waterdog Necturus alabamensis Central Newt Notophthalmus viridescens louisianensis Slimy Salamander Plethodon glutinosus glutinosus Slender Dwarf Siren Pseudobranchus striatus spheniscus Gulf Coast Mud Salamander Pseudotriton montanus flavissimus Southern Red Salamander Pseudotriton ruber vioscai Eastern Lesser Siren Siren intermedia intermedia Greater Siren Siren lacertina Toads Oak Toad Bufo quercicus Southern Toad Bufo terrestris Eastern Spadefoot Toad Scaphiopus holbrooki holbrooki Frogs Florida Cricket Frog Acris gryllus dorsalis Eastern Narrow-mouthed Frog Gastrophryne carolinensis Western Bird-voiced Treefrog Hyla avivoca avivoca Cope's Gray Treefrog Hyla chrysoscelis Green Treefrog Hyla cinerea Southern Spring Peeper Hyla crucifer bartramiana Pine Woods Treefrog Hyla femoralis Barking Treefrog Hyla gratiosa Squirrel Treefrog Hyla squirella Gray Treefrog Hyla versicolor Little Grass Frog Limnaoedus ocularis Southern Chorus Frog Pseudacris nigrita nigrita Ornate Chorus Frog Pseudacris -

Buttonbush, Cephalanthus Occidentalis
Buttonbush, Cephalanthus occidentalis Buttonbush is a deciduous, multi-stemmed, loose shrub or small tree in the coffee family (Rubiaceae). Generally reaching no more than 12 feet in height, the plant is frequently wider than it is tall. It is native to North America from Nova Scotia and Ontario south to Mexico. It is found in the Florida Everglades. A key characteristic of the Buttonbush is its flower heads which consist of fragrant, tiny, tubular, white flowers compacted into 1-inch diameter spheres. Each flower has a projecting style which gives the flower head a pincushion-like appearance. The long- lasting blooms (June – August) give way to hard, spherical clusters of nutlets that resemble old-time dress buttons; hence its common name. The fruit matures in fall and can stay on the plant through winter, providing interest for the garden and food for wildlife. The beautiful dark green, glossy foliage is another ornamental feature of Buttonbush. The leaves, which emerge late in spring, are opposite or in whorls of three, ovate in shape, 2-6 inches long, 1-3 inches wide, with a smooth edge and short petiole. The leaves turn yellow- green in the fall. Other identifying features of Buttonbush are prominent lenticels on coarse stems, absent terminal buds, and pith that is solid and light brown. Buttonbush occurs as a non-dominant midstory species in mixed riparian forests, pond or stream margins, and swamps. They prefer moist, humusy soils and full to partial sunlight. Buttonbush in the wild is an indicator of an area’s wetland status. The plant will tolerate water depths up to three feet and long durations of flooding. -

Number 3, Spring 1998 Director’S Letter
Planning and planting for a better world Friends of the JC Raulston Arboretum Newsletter Number 3, Spring 1998 Director’s Letter Spring greetings from the JC Raulston Arboretum! This garden- ing season is in full swing, and the Arboretum is the place to be. Emergence is the word! Flowers and foliage are emerging every- where. We had a magnificent late winter and early spring. The Cornus mas ‘Spring Glow’ located in the paradise garden was exquisite this year. The bright yellow flowers are bright and persistent, and the Students from a Wake Tech Community College Photography Class find exfoliating bark and attractive habit plenty to photograph on a February day in the Arboretum. make it a winner. It’s no wonder that JC was so excited about this done soon. Make sure you check of themselves than is expected to seedling selection from the field out many of the special gardens in keep things moving forward. I, for nursery. We are looking to propa- the Arboretum. Our volunteer one, am thankful for each and every gate numerous plants this spring in curators are busy planting and one of them. hopes of getting it into the trade. preparing those gardens for The magnolias were looking another season. Many thanks to all Lastly, when you visit the garden I fantastic until we had three days in our volunteers who work so very would challenge you to find the a row of temperatures in the low hard in the garden. It shows! Euscaphis japonicus. We had a twenties. There was plenty of Another reminder — from April to beautiful seven-foot specimen tree damage to open flowers, but the October, on Sunday’s at 2:00 p.m. -

Patterns in Ericaceae: New Phylogenetic Analyses
BS 55 479 Origins and biogeographic patterns in Ericaceae: New insights from recent phylogenetic analyses Kathleen A. Kron and James L. Luteyn Kron, KA. & Luteyn, J.L. 2005. Origins and biogeographic patterns in Ericaceae: New insights from recent phylogenetic analyses. Biol. Skr. 55: 479-500. ISSN 0366-3612. ISBN 87-7304-304-4. Ericaceae are a diverse group of woody plants that span the temperate and tropical regions of the world. Previous workers have suggested that Ericaceae originated in Gondwana, but recent phy logenetic studies do not support this idea. The theory of a Gondwanan origin for the group was based on the concentration of species richness in the Andes, southern Africa, and the southwest Pacific islands (most of which are thought to be of Gondwanan origin). The Andean diversity is comprised primarily of Vaccinieae with more than 800 species occurring in northern South America, Central America, and the Antilles. In the Cape Region of South Africa the genus Erica is highly diverse with over 600 species currently recognized. In the southwest Pacific islands, Vac cinieae and Rhodoreae are very diverse with over 290 species of Rhododendron (sect. Vireya) and approximately 500 species of Vaccinieae (I)imorphanthera, Paphia, Vaccinium). Recent phyloge netic studies have also shown that the Styphelioideae (formerly Epacridaceae) are included within Ericaceae, thus adding a fourth extremely diverse group (approximately 520 species) in areas considered Gondwanan in origin. Phylogenetic studies of the family on a global scale, how ever, have indicated that these highly diverse “Gondwanan” groups are actually derived from within Ericaceae. Both Fitch parsimony character optimization (using MacClade 4.0) and disper- sal-vicariance analysis (DIVA) indicate that Ericaceae is Laurasian in origin. -

Button Bush Cephalanthus Occidentalis L
W&M ScholarWorks Reports 11-1-1994 Button Bush Cephalanthus occidentalis L. Gene Silberhorn Virginia Institute of Marine Science Follow this and additional works at: https://scholarworks.wm.edu/reports Part of the Plant Sciences Commons Recommended Citation Silberhorn, G. (1994) Button Bush Cephalanthus occidentalis L.. Wetland Flora Technical Reports, Wetlands Program, Virginia Institute of Marine Science. Virginia Institute of Marine Science, College of William and Mary. http://dx.doi.org/doi:10.21220/m2-9xjm-rh51 This Report is brought to you for free and open access by W&M ScholarWorks. It has been accepted for inclusion in Reports by an authorized administrator of W&M ScholarWorks. For more information, please contact [email protected]. Wetlands Technical Report Program Wetland Flora No. 94-10 / November 1994 Gene Silberhorn Button Bush Cephalanthus occidentalis L. Growth Habit and Diagnostic Characteristics Habitat Button bush is a broad-leaved, deciduous shrub that Button bush may occupy several different types of grows up to 2 meters tall with an open spreading wetland habitats, including tidal and nontidal canopy. The simple, smooth marginate leaves are marshes, scrub/shrub and forested wetlands, and the usually oppositely arranged throughout the lower margins of lakes, ponds, ditches and streams. In branches, and are typically whorled (3 or 4 leaves at a bottomland, hardwood forests dominated by tupelo node) just below the terminal borne fruit or flowering (Nyssa aquatica) and bald cypress, Cephalanthus heads. Leaf petioles are often red during the peak occidentalis is often associated with other hydrophytic flowering period when the white globose heads shrubs such as swamp rose (Rosa palustris) and alder develop in July and August. -

Dictionary of Cultivated Plants and Their Regions of Diversity Second Edition Revised Of: A.C
Dictionary of cultivated plants and their regions of diversity Second edition revised of: A.C. Zeven and P.M. Zhukovsky, 1975, Dictionary of cultivated plants and their centres of diversity 'N -'\:K 1~ Li Dictionary of cultivated plants and their regions of diversity Excluding most ornamentals, forest trees and lower plants A.C. Zeven andJ.M.J, de Wet K pudoc Centre for Agricultural Publishing and Documentation Wageningen - 1982 ~T—^/-/- /+<>?- •/ CIP-GEGEVENS Zeven, A.C. Dictionary ofcultivate d plants andthei rregion so f diversity: excluding mostornamentals ,fores t treesan d lowerplant s/ A.C .Zeve n andJ.M.J ,d eWet .- Wageninge n : Pudoc. -11 1 Herz,uitg . van:Dictionar y of cultivatedplant s andthei r centreso fdiversit y /A.C .Zeve n andP.M . Zhukovsky, 1975.- Me t index,lit .opg . ISBN 90-220-0785-5 SISO63 2UD C63 3 Trefw.:plantenteelt . ISBN 90-220-0785-5 ©Centre forAgricultura l Publishing and Documentation, Wageningen,1982 . Nopar t of thisboo k mayb e reproduced andpublishe d in any form,b y print, photoprint,microfil m or any othermean swithou t written permission from thepublisher . Contents Preface 7 History of thewor k 8 Origins of agriculture anddomesticatio n ofplant s Cradles of agriculture and regions of diversity 21 1 Chinese-Japanese Region 32 2 Indochinese-IndonesianRegio n 48 3 Australian Region 65 4 Hindustani Region 70 5 Central AsianRegio n 81 6 NearEaster n Region 87 7 Mediterranean Region 103 8 African Region 121 9 European-Siberian Region 148 10 South American Region 164 11 CentralAmerica n andMexica n Region 185 12 NorthAmerica n Region 199 Specieswithou t an identified region 207 References 209 Indexo fbotanica l names 228 Preface The aimo f thiswor k ist ogiv e thereade r quick reference toth e regionso f diversity ofcultivate d plants.Fo r important crops,region so fdiversit y of related wild species areals opresented .Wil d species areofte nusefu l sources of genes to improve thevalu eo fcrops .