Evolutionary Patterns of Alternative Life Histories in Fishes: Assessing Marine-Freshwater and Aquatic-Terrestrial Movement
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

A Global Assessment of Parasite Diversity in Galaxiid Fishes
diversity Article A Global Assessment of Parasite Diversity in Galaxiid Fishes Rachel A. Paterson 1,*, Gustavo P. Viozzi 2, Carlos A. Rauque 2, Verónica R. Flores 2 and Robert Poulin 3 1 The Norwegian Institute for Nature Research, P.O. Box 5685, Torgarden, 7485 Trondheim, Norway 2 Laboratorio de Parasitología, INIBIOMA, CONICET—Universidad Nacional del Comahue, Quintral 1250, San Carlos de Bariloche 8400, Argentina; [email protected] (G.P.V.); [email protected] (C.A.R.); veronicaroxanafl[email protected] (V.R.F.) 3 Department of Zoology, University of Otago, P.O. Box 56, Dunedin 9054, New Zealand; [email protected] * Correspondence: [email protected]; Tel.: +47-481-37-867 Abstract: Free-living species often receive greater conservation attention than the parasites they support, with parasite conservation often being hindered by a lack of parasite biodiversity knowl- edge. This study aimed to determine the current state of knowledge regarding parasites of the Southern Hemisphere freshwater fish family Galaxiidae, in order to identify knowledge gaps to focus future research attention. Specifically, we assessed how galaxiid–parasite knowledge differs among geographic regions in relation to research effort (i.e., number of studies or fish individuals examined, extent of tissue examination, taxonomic resolution), in addition to ecological traits known to influ- ence parasite richness. To date, ~50% of galaxiid species have been examined for parasites, though the majority of studies have focused on single parasite taxa rather than assessing the full diversity of macro- and microparasites. The highest number of parasites were observed from Argentinean galaxiids, and studies in all geographic regions were biased towards the highly abundant and most widely distributed galaxiid species, Galaxias maculatus. -

The Role of Dimethyl Sulfoxide in the Cryopreservation of Curimba (Prochilodus Lineatus) Semen Semina: Ciências Agrárias, Vol
Semina: Ciências Agrárias ISSN: 1676-546X [email protected] Universidade Estadual de Londrina Brasil Varela Junior, Antonio Sergio; Fernandes Silva, Estela; Figueiredo Cardoso, Tainã; Yokoyama Namba, Érica; Desessards Jardim, Rodrigo; Streit Junior, Danilo Pedro; Dahl Corcini, Carine The role of dimethyl sulfoxide in the cryopreservation of Curimba (Prochilodus lineatus) semen Semina: Ciências Agrárias, vol. 36, núm. 5, septiembre-octubre, 2015, pp. 3471-3479 Universidade Estadual de Londrina Londrina, Brasil Available in: http://www.redalyc.org/articulo.oa?id=445744151041 How to cite Complete issue Scientific Information System More information about this article Network of Scientific Journals from Latin America, the Caribbean, Spain and Portugal Journal's homepage in redalyc.org Non-profit academic project, developed under the open access initiative DOI: 10.5433/1679-0359.2015v36n5p3471 The role of dimethyl sulfoxide in the cryopreservation of Curimba (Prochilodus lineatus) semen O papel do dimetilsulfóxido na criopreservação de sêmen de Curimba ( Prochilodus lineatus ) Antonio Sergio Varela Junior 1; Estela Fernandes Silva 2; Tainã Figueiredo Cardoso 2; Érica Yokoyama Namba 3; Rodrigo Desessards Jardim 1; Danilo Pedro Streit Junior 4; Carine Dahl Corcini 5* Abstract Cryopreservation of Curimba semen (Prochilodus lineatus) is ecological and commercial importance. The objective of this study was to evaluate the effect of different concentrations (2, 5, 8 and 11%) of dimethyl sulfoxide (DMSO) diluted in Betsville Thawing Solution (BTS) on the quality of post-thaw semen Curimba. We analyzed the rate and period motility, sperm viability, membrane integrity and DNA, mitochondrial functionality, and fertilization and hatching rate. The plasma membrane and DNA integrity of a DMSO concentration of 11% obtained better results than the concentration of 5% (p <0.05). -

FOOD and the FEEDING ECOLOGY of the MUDSKIPPERS (Periopthalmus Koelreutes (PALLAS) GOBODAC at RUMUOLUMENI CREEK, NIGER DELTA
AGRICULTURE AND BIOLOGY JOURNAL OF NORTH AMERICA ISSN Print: 2151-7517, ISSN Online: 2151-7525, doi:10.5251/abjna.2011.2.6.897.901 © 2011, ScienceHuβ, http://www.scihub.org/ABJNA Food and feeding ecology of the MUDSKIPPER Periopthalmus koelreuteri (PALLAS) Gobiidae at Rumuolumeni Creek, Niger Delta, Nigeria F.G. Bob-Manuel Rivers State University of Education, Rumuolumeni, P. M. B. 5047, Port Harcourt, Nigeria ABSTRACT The food habits of the mudskipper Periophthalmus keolreuteri (Pallas) from the mudflats at Rumuolumeni Creek, Niger Delta, Nigeria were studied. The frequency of occurrence and ‘point’ methods was used for the gut content analysis. The results indicate that the juveniles were herbivorous feeding more on aquatic macrophytes, diatoms and algal filaments while the adults had a dietary shift towards crustaceans, aquatic and terrestrial insects and polychaetes. The amphibious lifestyle of the mudskipper confers on it the trophic position of a zoobenthivore and a predator. Keywords: diet composition, mudskippers, food web, trophic relations, Niger Delta. INTRODUCTION catch bigger fishes. The rich food supplies in the mangrove mud flat have been the impetus which led The mudskippers Periophthalmus koelreuteri the goby-like ancestors of modern mudskippers to (Gobiidae: Oxudercinae) live in the intertidal habitat leave the water from time to time. (Evans et al., 1999) of the mudflats and in mangrove ecosystem (Murdy, The mudskippers can move rapidly on the mud, 1989). These fishes are uniquely adapted to a which is inaccessible to people. completely amphibious lifestyle (Graham, 1997). They are quite active when out of water, feeding and The mudskipper, P. koelreuteri is a residential fish interacting with one another and defending their inhabiting the mudflats of the Niger Delta estuaries, territories. -

Ecology, Biology and Taxonomy. Mudskippers
Chapter of the edited collection: Mangroves: Ecology, Biology and Taxonomy. Mudskippers: human use, ecotoxicology and biomonitoring of mangrove and other soft bottom intertidal ecosystems. Gianluca Polgar 1 and Richard Lim 2 1Institute of Biological Sciences, Institute of Ocean and Earth Sciences, Faculty of Science, University of Malaya, 50603 Kuala Lumpur, Malaysia. Tel. +603-7967-4609 / 4182; e-mail: [email protected] / [email protected]. Web site: www.themudskipper.org 2Centre for Environmental Sustainability, School of the Environment, Faculty of Science, University of Technology Sydney, Broadway, New South Wales 2007, Australia. e-mail: [email protected] Abstract (269) Mudskippers (Gobiidae: Oxudercinae) are air-breathing gobies, which are widely distributed throughout the West African coast and the Indo-Pacific region. They are closely linked to mangrove and adjacent soft bottom peri-tidal ecosystems. Some species are amongst the best adapted fishes to an amphibious lifestyle. All mudskippers are benthic burrowers in anoxic sediments, and since tidal mudflats are efficient sediment traps, and sinks for nutrients and other chemical compounds, they are constantly in contact with several types of pollutants produced by industrial, agricultural and domestic activities. Due to their natural abundance, considerable resistance to highly polluted conditions, and their benthic habits, mudskippers are frequently used in aquatic ecotoxicological studies. For the same reasons, mudskippers also frequently occur in urbanised or semi-natural coastal areas. Since several species are widely consumed throughout their whole geographical range, these same characteristics also facilitate their aquaculture in several countries, such as Bangladesh, Thailand, Philippines, China, Taiwan and Japan. Even when not directly used, mudskippers are often abundant and are important prey items for many intertidal transient species (marine visitors), and several species of shorebirds. -

Structure of Tropical River Food Webs Revealed by Stable Isotope Ratios
OIKOS 96: 46–55, 2002 Structure of tropical river food webs revealed by stable isotope ratios David B. Jepsen and Kirk O. Winemiller Jepsen, D. B. and Winemiller, K. O. 2002. Structure of tropical river food webs revealed by stable isotope ratios. – Oikos 96: 46–55. Fish assemblages in tropical river food webs are characterized by high taxonomic diversity, diverse foraging modes, omnivory, and an abundance of detritivores. Feeding links are complex and modified by hydrologic seasonality and system productivity. These properties make it difficult to generalize about feeding relation- ships and to identify dominant linkages of energy flow. We analyzed the stable carbon and nitrogen isotope ratios of 276 fishes and other food web components living in four Venezuelan rivers that differed in basal food resources to determine 1) whether fish trophic guilds integrated food resources in a predictable fashion, thereby providing similar trophic resolution as individual species, 2) whether food chain length differed with system productivity, and 3) how omnivory and detritivory influenced trophic structure within these food webs. Fishes were grouped into four trophic guilds (herbivores, detritivores/algivores, omnivores, piscivores) based on literature reports and external morphological characteristics. Results of discriminant function analyses showed that isotope data were effective at reclassifying individual fish into their pre-identified trophic category. Nutrient-poor, black-water rivers showed greater compartmentalization in isotope values than more productive rivers, leading to greater reclassification success. In three out of four food webs, omnivores were more often misclassified than other trophic groups, reflecting the diverse food sources they assimilated. When fish d15N values were used to estimate species position in the trophic hierarchy, top piscivores in nutrient-poor rivers had higher trophic positions than those in more productive rivers. -
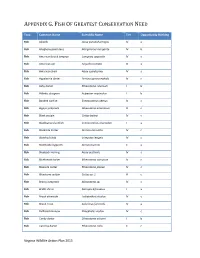
Fish of Greatest Conservation Need
APPENDIX G. FISH OF GREATEST CONSERVATION NEED Taxa Common Name Scientific Name Tier Opportunity Ranking Fish Alewife Alosa pseudoharengus IV a Fish Allegheny pearl dace Margariscus margarita IV b Fish American brook lamprey Lampetra appendix IV c Fish American eel Anguilla rostrata III a Fish American shad Alosa sapidissima IV a Fish Appalachia darter Percina gymnocephala IV c Fish Ashy darter Etheostoma cinereum I b Fish Atlantic sturgeon Acipenser oxyrinchus I b Fish Banded sunfish Enneacanthus obesus IV c Fish Bigeye jumprock Moxostoma ariommum III c Fish Black sculpin Cottus baileyi IV c Fish Blackbanded sunfish Enneacanthus chaetodon I a Fish Blackside darter Percina maculata IV c Fish Blotched chub Erimystax insignis IV c Fish Blotchside logperch Percina burtoni II a Fish Blueback Herring Alosa aestivalis IV a Fish Bluebreast darter Etheostoma camurum IV c Fish Blueside darter Etheostoma jessiae IV c Fish Bluestone sculpin Cottus sp. 1 III c Fish Brassy Jumprock Moxostoma sp. IV c Fish Bridle shiner Notropis bifrenatus I a Fish Brook silverside Labidesthes sicculus IV c Fish Brook Trout Salvelinus fontinalis IV a Fish Bullhead minnow Pimephales vigilax IV c Fish Candy darter Etheostoma osburni I b Fish Carolina darter Etheostoma collis II c Virginia Wildlife Action Plan 2015 APPENDIX G. FISH OF GREATEST CONSERVATION NEED Fish Carolina fantail darter Etheostoma brevispinum IV c Fish Channel darter Percina copelandi III c Fish Clinch dace Chrosomus sp. cf. saylori I a Fish Clinch sculpin Cottus sp. 4 III c Fish Dusky darter Percina sciera IV c Fish Duskytail darter Etheostoma percnurum I a Fish Emerald shiner Notropis atherinoides IV c Fish Fatlips minnow Phenacobius crassilabrum II c Fish Freshwater drum Aplodinotus grunniens III c Fish Golden Darter Etheostoma denoncourti II b Fish Greenfin darter Etheostoma chlorobranchium I b Fish Highback chub Hybopsis hypsinotus IV c Fish Highfin Shiner Notropis altipinnis IV c Fish Holston sculpin Cottus sp. -

Note Food and Feeding Habits of Macrobrachium
Indian J. Fish., 60(4) : 131-135, 2013 Note Food and feeding habits of Macrobrachium lar (Decapoda, Palaemonidae) from Andaman and Nicobar Islands, India S. N. SETHI1, NAGESH RAM2 AND V. VENKATESAN3 1Chennai Research Centre of Central Marine Fisheries Research Institute, 75 – Santhome High Road R. A. Puram, Chennai-600 028,Tamil Nadu, India 2Central Agricultural Research Institute (CARI), Port Blair-744 103, Andaman and Nicobar Islands, India 3Central Marine Fisheries Research Institute, Cochin -682 018, Kerala, India e-mail: [email protected] ABSTRACT The stomach content of the freshwater prawn Macrobrachium lar from the inland water bodies of Andaman and Nicobar Islands were analysed in relation to size, sex and season during the period from January to December 2008. Feeding habits of M. lar were studied using the index of preponderance and feeding intensity methods. Mainly organic detritus, supplemented by animal and plant materials formed the food in all stages of M. lar. The animal food items mainly consisted of aquatic insects, polychaetes, other crustaceans, fish, molluscs and zooplankton. The plant matter was chiefly composed of fragments of aquatic plants, planktonic algae and diatoms. Variation in diet in relation to size, sex or season were found insignificant. Though, all size groups of this species feed animal and plant materials, it exhibited slight preference for animal food especially in larger size groups. Females appear to feed more actively than the males. Feeding intensity was more in monsoon season compared to dry season. Results indicate that the adults are mostly predators of slow moving benthic invertebrates rather than detritus feeders/scavengers. -

Inland Waters
477 Fish, crustaceans, molluscs, etc Capture production by species items America, South - Inland waters C-03 Poissons, crustacés, mollusques, etc Captures par catégories d'espèces Amérique du Sud - Eaux continentales (a) Peces, crustáceos, moluscos, etc Capturas por categorías de especies América del Sur - Aguas continentales English name Scientific name Species group Nom anglais Nom scientifique Groupe d'espèces 2012 2013 2014 2015 2016 2017 2018 Nombre inglés Nombre científico Grupo de especies t t t t t t t Common carp Cyprinus carpio 11 321 114 134 179 169 46 240 Cyprinids nei Cyprinidae 11 425 429 423 400 400 400 400 ...A Caquetaia kraussii 12 ... ... 11 182 111 559 64 Nile tilapia Oreochromis niloticus 12 5 7 3 6 255 257 159 Tilapias nei Oreochromis (=Tilapia) spp 12 9 133 9 210 9 093 8 690 8 600 8 600 8 600 Oscar Astronotus ocellatus 12 1 847 1 862 1 951 1 941 1 825 1 813 1 815 Velvety cichlids Astronotus spp 12 391 385 318 571 330 345 334 Green terror Aequidens rivulatus 12 26 38 20 24 36 30 34 Cichlids nei Cichlidae 12 13 013 13 123 12 956 12 400 12 403 12 735 12 428 Arapaima Arapaima gigas 13 1 478 1 504 1 484 2 232 1 840 2 441 1 647 Arawana Osteoglossum bicirrhosum 13 1 642 1 656 1 635 1 570 1 571 2 200 2 056 Banded astyanax Astyanax fasciatus 13 1 043 1 052 1 039 1 000 1 000 1 000 1 000 ...A Brycon orbignyanus 13 8 8 8 8 8 9 14 ...A Brycon dentex 13 35 20 5 6 11 10 6 ...A Brycon spp 13 .. -

Karyotypic Characterization of Prochilodus Mariae, Semaprochilodus Kneri Ands. Laticeps (Teleostei: Prochilodontidae) from Caica
Neotropical Ichthyology, 1(1):47-52, 2003 Copyright © 2003 Sociedade Brasileira de Ictiologia Karyotypic characterization of Prochilodus mariae, Semaprochilodus kneri and S. laticeps (Teleostei: Prochilodontidae) from Caicara del Orinoco, Venezuela Claudio Oliveira*, Mauro Nirchio**, Ángel Granado*** and Sara Levy** Fish of the family Prochilodontidae are considered one of the most important components of commercial and subsistence fishery in freshwater environments in South America. This family consists of 21 species and three genera. In the present study, the karyotypes of Prochilodus mariae, Semaprochilodus kneri, and S. laticeps from Caicara del Orinoco, Bolivar State, Venezuela were studied. The species P. mariae, S. kneri and S. laticeps exhibited 2n=54 chromosomes (40 metacentric and 14 submetacentric), a single chromosome pair with nucleolus organizer regions, and a large amount of heterochromatin found at centromeric and pericentromeric positions in almost all chromosomes. The P. mariae specimens studied displayed 0 to 3 supernumerary microchromosomes. The data obtained here confirm the conservative nature of the chromosome number and morphology of Prochilodontidae and reinforce the hypothesis that small structural chromosome rearrangements were the main cause of the karyotypic diversification seen in this group. Os peixes da família Prochilodontidae são considerados um dos componentes mais importantes da pesca comercial e de subsistência em ambientes de água doce na América do Sul. Essa família compreende 21 espécies e três gêneros. No presente estudo foram analisados os cariótipos de Prochilodus mariae, Semaprochilodus kneri e S. laticeps provenientes de Caicara del Orinoco, Estado Bolivar, Venezuela. As espécies P. mariae, S. kneri e S. laticeps apresentaram 2n=54 cromossomos (40 metacêntricos e 14 submetacêntricos), um único par de cromossomos com regiões organizadoras de nucléolo e uma grande quantidade de heterocromatina em posição centromérica e pericentromérica de quase todos os cromossomos. -

Candy Darter Recovery Outline
U.S. Fish & Wildlife Service Candy Darter Recovery Outline Photo by Corey Dunn, University of Missouri Species Name: Candy Darter (Etheostoma osburni) Species Range: Upper Kanawha River Basin including the Gauley, Greenbrier, and New River Watersheds including portions of Greenbrier, Pocahontas, Nicholas, and Webster counties in West Virginia; and Bland, Giles, and Wythe counties, Virginia. The range of the species is shown in figure 1 below. Recovery Priority Number: 5; explanation provided below Listing Status: Endangered; November 21, 2019; 83 FR 58747 Lead Regional Office/Cooperating RO(s): Northeast Region, Hadley MA Lead Field Office/Cooperating FO(s): West Virginia Field Office, Elkins WV; Southwestern Virginia Field Office, Abingdon, VA; White Sulphur Springs National Fish Hatchery, White Sulphur Springs, WV Lead Contact: Barbara Douglas, 304-636-6586 ext 19. [email protected] 1) Background This section provides a brief overview of the ecology and conservation of the candy darter. This information is more fully described in the Species Status Assessment (SSA), the final listing rule, and the proposed critical habitat rule. These documents are available at: https://www.fws.gov/northeast/candydarter/ 1 Type and Quality of Available Information to Date: Important Information Gaps and Treatment of Uncertainties One of the primary threats that resulted in the listing of the candy darter under the Endangered Species Act (ESA) is the spread of the introduced variegate darter (Etheostoma variatum). This species hybridizes with the candy darter. Key information gaps and areas of uncertainty for the recovery and management of the candy darter include whether there are any habitat or other natural factors that could limit the spread of variegate darter. -

Aquatic Ecology of the Montagu River Catchment
Aquatic Ecology of the Montagu River Catchment A Report Forming Part of the Requirements for State of Rivers Reporting David Horner Water Assessment and Planning Branch Water Resources Division DPIWE. December, 2003 State of Rivers Aquatic Ecology of the Montagu Catchment Copyright Notice: Material contained in the report provided is subject to Australian copyright law. Other than in accordance with the Copyright Act 1968 of the Commonwealth Parliament, no part of this report may, in any form or by any means, be reproduced, transmitted or used. This report cannot be redistributed for any commercial purpose whatsoever, or distributed to a third party for such purpose, without prior written permission being sought from the Department of Primary Industries, Water and Environment, on behalf of the Crown in Right of the State of Tasmania. Disclaimer: Whilst DPIWE has made every attempt to ensure the accuracy and reliability of the information and data provided, it is the responsibility of the data user to make their own decisions about the accuracy, currency, reliability and correctness of information provided. The Department of Primary Industries, Water and Environment, its employees and agents, and the Crown in the Right of the State of Tasmania do not accept any liability for any damage caused by, or economic loss arising from, reliance on this information. Preferred Citation: DPIWE (2003). State of the River Report for the Montagu River Catchment. Water Assessment and Planning Branch, Department of Primary Industries, Water and Environment, Hobart. Technical Report No. WAP 03/09 ISSN: 1449-5996 The Department of Primary Industries, Water and Environment The Department of Primary Industries, Water and Environment provides leadership in the sustainable management and development of Tasmania’s resources. -

Proceedings of the Indiana Academy of Science 1 1 8(2): 143—1 86
2009. Proceedings of the Indiana Academy of Science 1 1 8(2): 143—1 86 THE "LOST" JORDAN AND HAY FISH COLLECTION AT BUTLER UNIVERSITY Carter R. Gilbert: Florida Museum of Natural History, University of Florida, Gainesville, Florida 32611 USA ABSTRACT. A large fish collection, preserved in ethanol and assembled by Drs. David S. Jordan and Oliver P. Hay between 1875 and 1892, had been stored for over a century in the biology building at Butler University. The collection was of historical importance since it contained some of the earliest fish material ever recorded from the states of South Carolina, Georgia, Mississippi and Kansas, and also included types of many new species collected during the course of this work. In addition to material collected by Jordan and Hay, the collection also included specimens received by Butler University during the early 1880s from the Smithsonian Institution, in exchange for material (including many types) sent to that institution. Many ichthyologists had assumed that Jordan, upon his departure from Butler in 1879. had taken the collection. essentially intact, to Indiana University, where soon thereafter (in July 1883) it was destroyed by fire. The present study confirms that most of the collection was probably transferred to Indiana, but that significant parts of it remained at Butler. The most important results of this study are: a) analysis of the size and content of the existing Butler fish collection; b) discovery of four specimens of Micropterus coosae in the Saluda River collection, since the species had long been thought to have been introduced into that river; and c) the conclusion that none of Jordan's 1878 southeastern collections apparently remain and were probably taken intact to Indiana University, where they were lost in the 1883 fire.