People, Pollution and Pathogens – Global Change Impacts in Mountain Freshwater Ecosystems
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

The Genus Vaccinium in North America
Agriculture Canada The Genus Vaccinium 630 . 4 C212 P 1828 North America 1988 c.2 Agriculture aid Agri-Food Canada/ ^ Agnculturo ^^In^iikQ Canada V ^njaian Agriculture Library Brbliotheque Canadienno de taricakun otur #<4*4 /EWHE D* V /^ AgricultureandAgri-FoodCanada/ '%' Agrrtur^'AgrntataireCanada ^M'an *> Agriculture Library v^^pttawa, Ontano K1A 0C5 ^- ^^f ^ ^OlfWNE D£ W| The Genus Vaccinium in North America S.P.VanderKloet Biology Department Acadia University Wolfville, Nova Scotia Research Branch Agriculture Canada Publication 1828 1988 'Minister of Suppl) andS Canada ivhh .\\ ailabla in Canada through Authorized Hook nta ami other books! or by mail from Canadian Government Publishing Centre Supply and Services Canada Ottawa, Canada K1A0S9 Catalogue No.: A43-1828/1988E ISBN: 0-660-13037-8 Canadian Cataloguing in Publication Data VanderKloet,S. P. The genus Vaccinium in North America (Publication / Research Branch, Agriculture Canada; 1828) Bibliography: Cat. No.: A43-1828/1988E ISBN: 0-660-13037-8 I. Vaccinium — North America. 2. Vaccinium — North America — Classification. I. Title. II. Canada. Agriculture Canada. Research Branch. III. Series: Publication (Canada. Agriculture Canada). English ; 1828. QK495.E68V3 1988 583'.62 C88-099206-9 Cover illustration Vaccinium oualifolium Smith; watercolor by Lesley R. Bohm. Contract Editor Molly Wolf Staff Editors Sharon Rudnitski Frances Smith ForC.M.Rae Digitized by the Internet Archive in 2011 with funding from Agriculture and Agri-Food Canada - Agriculture et Agroalimentaire Canada http://www.archive.org/details/genusvacciniuminOOvand -
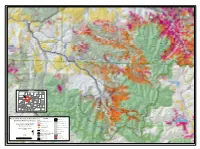
Forest Wide Hazardous Tree Removal and Fuels Reduction Project
107°0'0"W VAIL k GYPSUM B e 6 u 6 N 1 k 2 k 1 h 2 e . e 6 . .1 I- 1 o 8 70 e c f 7 . r 0 e 2 2 §¨¦ e l 1 0 f 2 u 1 0 3 2 N 4 r r 0 1 e VailVail . 3 W . 8 . 1 85 3 Edwards 70 1 C 1 a C 1 .1 C 8 2 h N 1 G 7 . 7 0 m y 1 k r 8 §¨¦ l 2 m 1 e c . .E 9 . 6 z W A T m k 1 5 u C 0 .1 u 5 z i 6. e s 0 C i 1 B a -7 k s 3 2 .3 e e r I ee o C r a 1 F G Carterville h r e 9. 1 6 r g 1 N 9 g 8 r e 8 r y P e G o e u l Avon n C 9 N C r e n 5 ch w i r 8 .k2 0 N n D k 1 n 70 a tt e 9 6 6 8 G . c 7 o h 18 1 §¨¦ r I-7 o ra West Vail .1 1 y 4 u h 0 1 0. n lc 7 l D .W N T 7 39 . 71 . 1 a u 1 ch W C k 0 C d . 2 e . r e 1 e 1 C st G e e . r 7 A Red Hill R 3 9 k n s e 5 6 7 a t 2 . -

Summits on the Air – ARM for USA - Colorado (WØC)
Summits on the Air – ARM for USA - Colorado (WØC) Summits on the Air USA - Colorado (WØC) Association Reference Manual Document Reference S46.1 Issue number 3.2 Date of issue 15-June-2021 Participation start date 01-May-2010 Authorised Date: 15-June-2021 obo SOTA Management Team Association Manager Matt Schnizer KØMOS Summits-on-the-Air an original concept by G3WGV and developed with G3CWI Notice “Summits on the Air” SOTA and the SOTA logo are trademarks of the Programme. This document is copyright of the Programme. All other trademarks and copyrights referenced herein are acknowledged. Page 1 of 11 Document S46.1 V3.2 Summits on the Air – ARM for USA - Colorado (WØC) Change Control Date Version Details 01-May-10 1.0 First formal issue of this document 01-Aug-11 2.0 Updated Version including all qualified CO Peaks, North Dakota, and South Dakota Peaks 01-Dec-11 2.1 Corrections to document for consistency between sections. 31-Mar-14 2.2 Convert WØ to WØC for Colorado only Association. Remove South Dakota and North Dakota Regions. Minor grammatical changes. Clarification of SOTA Rule 3.7.3 “Final Access”. Matt Schnizer K0MOS becomes the new W0C Association Manager. 04/30/16 2.3 Updated Disclaimer Updated 2.0 Program Derivation: Changed prominence from 500 ft to 150m (492 ft) Updated 3.0 General information: Added valid FCC license Corrected conversion factor (ft to m) and recalculated all summits 1-Apr-2017 3.0 Acquired new Summit List from ListsofJohn.com: 64 new summits (37 for P500 ft to P150 m change and 27 new) and 3 deletes due to prom corrections. -

Geology of the Pegmatites and Associated Rocks of Maine
DEPARTMENT OF THE INTERIOR UNITED STATES GEOLOGICAL SURVEY GEORGE OTIS SMITH, DIRECTOR BULLETIN 445 GEOLOGY OF THE PEGMATITES AND ASSOCIATED ROCKS OF MAINE INCLUDING FELDSPAR, QUARTZ, MICA, AND GEM DEPOSITS BY EDSON S. BASTIN WASHINGTON GOVERNMENT PRINTING OFFICE 1911 CONTENTS. Introduction.............................................................. 9 Definition of pegmatite...................................................... 10 Geographic distribution.................................................... 10 Geology.................................................................. 10 Bordering rocks....................................................... 10 Pegmatites in foliated rocks........................................ 11 General statement............................................ 11 Sedimentary foliates........................................... 11 Igneous foliates.....".......................................... 12 Pegmatites in massive granites.................................... 13 'Age.................................................................. 15 General character..................................................... 15 Mineral and chemical composition................................. 15 Mineral constituents.......................................... 15 Relative proportions of minerals............................... 18 Quartzose phases. ..............................^............. 18 Fluidal cavities............................................... 19 Sodium and lithium phases................................... 20 Muscovite -

¥¦70 $+9 $+6 $+7 ¥¦70
]" 77 3 6 023 60 2 32 3 .1 6 C N199 701 ! 73 1 4 70 7 0 0 0 ! ! 2 7 0 e r u t l u c i r g A f o t n e m t r a p e D s e t a t S d e t i n U 5 0 ! 7 3 1 02 6 7 290000 300000 310000 320000 330000 340000 350000 360000 7 370000 107°30'0"W 107°22'30"W 107°15'0"W 107°7'30"W 107°0'0"W 106°52'30"W 106°45'0"W 106°37'30"W 106°30'0"W ! e c i v r e S t s e r o F o d a r o l o C Blue !C ! Lake 7 ! Flatiron Mountain 1 E 7 ! R ! a 0 2 y a M - 3 2 v o N : s e t a D e v i t c e f f E i ! g f g Haypress l l l e ! e e Casteel Ridge 7 74 ! Lake R ! R Legend ! ! See Eagle/Holy Cross ! ! a a ! ! ! n n ! ! ! n n Gypsum !Winter Motor Vehicle Use Map Bellyache Ridge ! g g Dotsero g Plowed Route: g ! e e e e ! ]" r r ! 774 r Route maintained for winter r Eagle ! ! D 60 D D 2 D !C wheeled motor vehicle access 70 i i Eagle Ranger i i s 4390000 s Burnt Tree Ridge 4390000 s s t t ¥¦ Edwards t t r r District Office r r i i c c i i c c t 39°37'30"N t t Route shown in Open Motorized area t for informational purposes only 23 6 +$ +$25 ¤£ ! ! ! Designated Routes in Restricted Areas: Avon ]" Routes open to over-snow motorized Red Hill 307 n +$ 39°37'30"N vehicles where over-snow travel is yo an restricted to designated routes C d (100 feet either side) oo nw le !C G Motorized Prohibited Areas: Storm King Mountain 25A Holy Cross ]"!C Area where no motorized +$ Ranger District over-snow use is permitted 2 137 0 8A +$ 6 Spruce Ridge +$ Bellyache Mountain Office Horse Mountain Restricted Areas - Motorized RouteNs eOwnly C: astle Gobbler Knob North Hardscrabble Mountain Area where motorized over-snow ! 10A Minturn er Distr +$ 102 use is permitted only on designated ng ict +$ motorized routes. -

State of Maine Birding Map and Trail Guide
MAINE The Regions Lorem ipsum dolor of Maine sit amet, cosecte THE MAINE BEACHES taur adipisicing elit, Famous for beaches and amuse- seddor incididunt ments. Notable birds: Piping ut labore et dolore Plover, Least Tern, Harlequin magna ali qua. Duck, Upland Sandpiper. Lorem ipsum dol GREATER PORTLAND AND CASCO BAY sit amet, cosecte Famous for Maine’s largest adipisicing elit, city and Scarborough Marsh. seddor incididm. Notable birds: Roseate Tern and Sharp-tailed Sparrows. MID-COAST REGION Famous for extraordinary state parks, islands, and sailing. Notable birds include: Atlantic Puffin and Roseate Tern. DOWNEAST AND ACADIA Famous for Acadia National Park, national wildlife refuges, and state parks. Notable birds: Atlantic Puffin, Razorbill, and Spruce Grouse. MAINELAKES & MOUNTAINS Famous for scenic nature and solitude. Notable birds: Common Loon, Philadelphia Vireo, and Boreal Chickadee. KENNEBEC VALLEY Famous for hiking, skiing, and white-water-rafting. Notable birds: Warblers, w. e. Gray Jay, Crossbills, Bicknell’s Thrush. s. THEMAINEHIGHLANDS Famous for Moosehead Lake and Baxter State Park. Zone 1 Zone 2 Zone 3 Notable birds: Spruce Grouse, Black-backed Woodpecker. coastal and transition industrial river valley belt forest AROOSTOOK COUNTY lowlands mixed forest spruce/fir forest Famous for agriculture and Acadian beaches faster rivers undeveloped French tradition. Notable birds: rocky coastlines lakes shorelines American Three-toed Woodpecker, slow streams mountains remote recreation Pine Grosbeak, Crossbills. Lorem ipsum dolor sit amet, cosecte taur hardwood forests adipisicing elit, seddor incididunt ut lab LEGEND Vireo, Gray Jay, Boreal Chickadee, Bicknell’s Thrush, Laboris nisi liquip Contents and a variety of warblers. Both of the secretive Sharp-tailed Birding Trails Duis irure d lor repre hen Sparrow species are late nesters and are reasonably easy to Cillum dolore IN MAINE see through July. -

WAR UPON MEXICO US Army Mortality Statistics
GO TO MASTER INDEX OF WARFARE WAR UPON MEXICO US Army Mortality Statistics War on Mexico 110 per 1,000 Civil War 65 per 1,000 “I do not think there was ever waged a more wicked war than that waged by the United States on Mexico. I thought so at the time, when I was a youngster, only I had not moral courage enough to resign.” — Ulysses S. Grant, PERSONAL MEMOIRS, 1885 “Fiddle-dee-dee, war, war, war, I get so bored I could scream!” —Scarlet O’Hara Over the years, people I’ve met have often asked me what I’m working on, and I’ve usually replied that the main thing was a book about Dresden. I said that to Harrison Starr, the movie-maker, one time, and he raised his eyebrows and inquired, “Is it an anti-war book?” “Yes,” I said. “I guess.” “You know what I say to people when I hear they’re writing anti-war books?” “No. What do you say, Harrison Starr?” “I say, ‘Why don’t you write an anti-glacier book instead?’” What he meant, of course, was that there would always be wars, that they were as easy to stop as glaciers. I believe that, too. — Kurt Vonnegut, Jr. SLAUGHTERHOUSE-FIVE OR THE CHILDREN’S CRUSADE A DUTY-DANCE WITH DEATH. NY: Dell, 1971, page 3. HDT WHAT? INDEX MEXICO MEJICO GO TO MASTER INDEX OF WARFARE 1632 Bernal Diaz del Castillo’s THE CONQUEST OF MEXICO. 1810 October 19, day: Mexican revolutionary leader Father Miguel Hidalgo y Costilla proclaimed the end of slavery in the nation. -

Orogrande OHV Trail Project Environmental Assessment
United States Department of Agriculture Orogrande OHV Forest Service Trail Project March 2012 Environmental Assessment North Fork Ranger District Clearwater National Forest Orogrande OHV Trail Project Environmental Assessment Orogrande OHV Trail Project Environmental Assessment Orogrande OHV Trail Project Environmental Assessment North Fork Ranger District Clearwater National Forest Northern Region, USDA Forest Service March 2012 Responsible Agency: USDA Forest Service Responsible Official: Kathryn Rodriguez, District Ranger North Fork Ranger District 12730 Hwy 12 Orofino, ID 83544 For further information, contact: Tammy Harding, Interdisciplinary Team Leader Lochsa Ranger Station rd 903 3 Street Kamiah, ID 83536 “The U.S. Department of Agriculture (USDA) prohibits discrimination in all its programs and activities on the basis of race, color, national origin, age, disability, and where applicable, sex, marital status, familial status, parental status, religion, sexual orientation, genetic information, political beliefs, reprisal, or because all or part of an individual’s income is derived from any public assistance program. (Not all prohibited bases apply to all programs.) Persons with disabilities who require alternative means for communication of program information (Braille, large print, audiotape, etc.) should contact USDA's TARGET Center at (202) 720-2600 (voice and TDD). To file a complaint of discrimination, write to USDA, Director, Office of Civil Rights, 1400 Independence Avenue, S.W., Washington, D.C. 20250-9410, or call (800) 795- 3272 (voice) or (202)720-6382 (TDD). USDA is an equal opportunity provider and employer.” Orogrande OHV Trail Project Environmental Assessment Orogrande OHV Trail Project Environmental Assessment Table of Contents Page Number CHAPTER 1 PURPOSE AND NEED FOR ACTION Introduction . -

Summer 2020 COVID-19 Effects on Rides and Races Kevin Bessett
PerspectivesVolume 32 • Issue 2 Summer 2020 COVID-19 Effects on Rides and Races KEVIN BESSETT he last five months have been The COVID era has led to significant may happen in early August. The nothing short of surreal, strange, changes in how the club conducts Wednesday evening ride is not likely Tweird, scary, and thought rides. The first change is that you to happen this year. provoking. The world has more must pre-register online for each ride. than enough problems as it is, and This is for tracking purposes and I On the racing side, TTs may start in throwing a pandemic onto the heap of will delete the information collected the second half of July, but it is not challenges is not helping matters. after 30 days. You will find the looking promising for any practice details in the ride announcement crits this year. Here in Vermont we are fortunate on the Listserv. You must practice to have the virus mostly under distancing of at least six feet before, Please remember that rides/events control (let’s all try to keep it that during, and after rides. Lastly, we are dependent on two factors: way) and some outdoor activities ask that you carry a face mask or Volunteers and the State’s COVID-19 around the state have started up. covering on the ride. There are guidance. Some volunteers may Included in this are VP and touring several reasons for this. Some stores understandably feel uneasy about rides. Getting to this point required require masks, and if you have a the possible exposure to the virus, a surprising amount of work (tons of mechanical and need to get a ride and if replacements are not located, emails, research, website updates, back to your car, the driver will likely the club will cancel the event. -

The Sierra Club Pictorial Collections at the Bancroft Library Call Number Varies
The Sierra Club Pictorial Collections at The Bancroft Library Call Number Varies Chiefly: BANC PIC 1971.031 through BANC PIC 1971.038 and BANC PIC 1971.073 through 1971.120 The Bancroft Library U.C. Berkeley This is a DRAFT collection guide. It may contain errors. Some materials may be unavailable. Draft guides might refer to material whose location is not confirmed. Direct questions and requests to [email protected] Preliminary listing only. Contents unverified. Direct questions about availability to [email protected] The Sierra Club Pictorial Collections at The Bancroft Library Sierra Club Wilderness Cards - Series 1 BANC PIC 1971.026.001 ca. 24 items. DATES: 19xx Item list may be available at library COMPILER: Sierra Club DONOR: SIZE: PROVENANCE: GENERAL NOTE No Storage Locations: 1971.026.001--A Sierra Club Wilderness Cards - Series 1 24 items Index Terms: Places Represented Drakes Bay (Calif.) --A Echo Park, Dinosaur National Monument (Colo.) --A Northern Cascades (Wash.) --A Point Reyes (Calif.) --A Sawtooth Valley (Idaho) --A Sequoia National Forest (Calif.) --A Volcanic Cascades (Or.) --A Waldo Lake (Or.) --A Wind River (Wyo.) --A Photographer Blaisdell, Lee --A Bradley, Harold C. --A Brooks, Dick --A Douglas, Larry --A Faulconer,DRAFT Philip W. --A Heald, Weldon Fairbanks, 1901-1967 --A Hessey, Charles --A Hyde, Philip --A Litton, Martin --A Riley, James --A Simons, David R., (David Ralph) --A Tepfer, Sanford A. --A Warth, John --A Worth, Don --A Wright, Cedric --A Page 1 of 435 Preliminary listing only. Contents unverified. Direct questions about availability to [email protected] The Sierra Club Pictorial Collections at The Bancroft Library "Discover our outdoors" BANC PIC 1971.026.002 ca. -

T R a V E L P L a N N
United States Department of Agriculture T R A V E L P L A N N E R Forest Service CLEARWATER NEZ PERCE COUNTRY ★ Gospel-Hump Wilderness, Nez Perce National Forest A guide to recreational opportunities on public lands in north central Idaho WELCOM E! ROAD AND TRAIL ACCESS # No rth c e n tra l Id a h o is d iv e rs e , in te re s tin g a n d Many roads and trails on national forests are open b e a u tifu l. W e w e lc o m e y o u r to enjoy. Some modes of travel may be restricted to in te re s t in th e a re a . It is a protect areas sensitive for big-game animals like elk. p le a s u re to p ro v id e in fo r- Other times, roads and trails are closed during wet m a tio n to h e lp y o u p la n weather to prevent roadbed damage and erosion. In y o u r v is it. If y o u h a v e q u e s tio n s , p le a s e some specific instances, certain types of travel are c o n ta c t u s a t a n y C le a rw a te r o r Ne z restricted to insure public safety. A forest travel P e rc e Na tio n a l F o re s t o ffic e . -

Old Spanish Trail Association, Has Been in Existence for Several Years
Draft National Historic Trail Feasibility Study and Environmental Assessment July 2000 OLD SPANISH TRAIL New Mexico · Colorado · Utah · Arizona · Nevada · California United States Department of the Interior ? National Park Service Draft National Historic Trail Feasibility Study and Environmental Assessment July 2000 OLD SPANISH TRAIL New Mexico · Colorado · Utah · Arizona · Nevada · California United States Department of the Interior ? National Park Service ACKNOWLEDGEMENTS The National Park Service thanks the technical team and others who assisted in the preparation and review of this document. In the interest of historical accuracy, these people generously shared their knowledge of the history and resources of the Old Spanish Trail. The participation of these people has improved the document and will serve future generations well. SUMMARY The purpose of this study is to evaluate the feasibility and desirability of designating the Old Spanish Trail as a National Historic Trail under the study provisions of the National Trails System Act (Public Law 90-543, 16 USC 1241, et seq.). Pioneered by Mexican trader Antonio Armijo in 1829, the Old Spanish Trail was a horse and burro pack route that connected Santa Fe and Los Angeles. In its early years, trappers, slavers, traders, and immigrants used parts or all of the Old Spanish Trail. Other variants of the Old Spanish Trail developed as travelers sought adequate water, grazing, shorter distances, smoother terrain, and safer passage. Over time, multiple, parallel, and intertwined routes developed. Many of these routes followed older trails developed by American Indians, and were later followed by Spanish, Mexican, and other Euro- American explorers. After 1848, use of the eastern end of the trail diminished as the California Trail to the north and southern trails across Arizona became the primary routes to California.