Carbohydrates Structure and Classification Term Carbohydrate Is Derived from the French: HYDRATE DE CARBONE Compounds Composed of C, H, and O
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Postulated Physiological Roles of the Seven-Carbon Sugars, Mannoheptulose, and Perseitol in Avocado
J. AMER. SOC. HORT. SCI. 127(1):108–114. 2002. Postulated Physiological Roles of the Seven-carbon Sugars, Mannoheptulose, and Perseitol in Avocado Xuan Liu,1 James Sievert, Mary Lu Arpaia, and Monica A. Madore2 Department of Botany and Plant Sciences, University of California, Riverside, CA 92521 ADDITIONAL INDEX WORDS. ‘Hass’ avocado on ‘Duke 7’ rootstock, phloem transport, ripening, Lauraceae ABSTRACT. Avocado (Persea americana Mill.) tissues contain high levels of the seven-carbon (C7) ketosugar mannoheptulose and its polyol form, perseitol. Radiolabeling of intact leaves of ‘Hass’ avocado on ‘Duke 7’ rootstock indicated that both perseitol and mannoheptulose are not only primary products of photosynthetic CO2 fixation but are also exported in the phloem. In cell-free extracts from mature source leaves, formation of the C7 backbone occurred by condensation of a three-carbon metabolite (dihydroxyacetone-P) with a four-carbon metabolite (erythrose-4-P) to form sedoheptulose-1,7- bis-P, followed by isomerization to a phosphorylated D-mannoheptulose derivative. A transketolase reaction was also observed which converted five-carbon metabolites (ribose-5-P and xylulose-5-P) to form the C7 metabolite, sedoheptu- lose-7-P, but this compound was not metabolized further to mannoheptulose. This suggests that C7 sugars are formed from the Calvin Cycle, not oxidative pentose phosphate pathway, reactions in avocado leaves. In avocado fruit, C7 sugars were present in substantial quantities and the normal ripening processes (fruit softening, ethylene production, and climacteric respiration rise), which occurs several days after the fruit is picked, did not occur until levels of C7 sugars dropped below an apparent threshold concentration of ≈20 mg·g–1 fresh weight. -

Determination of Total Dietary Fibre and Available Carbohydrates: a Rapid Integrated Procedure That Simulates in Vivo Digestion
860 DOI 10.1002/star.201500017 Starch/Stärke 2015, 67, 860–883 RESEARCH ARTICLE Determination of total dietary fibre and available carbohydrates: A rapid integrated procedure that simulates in vivo digestion Barry V. McCleary, Naomi Sloane and Anna Draga Megazyme International Ireland, Bray Business Park, Bray, County Wicklow, Ireland The new definition of dietary fibre introduced by Codex Alimentarius in 2008 includes resistant Received: January 23, 2015 starch and the option to include non-digestible oligosaccharides. Implementation of this definition Revised: March 5, 2015 required new methodology. An integrated total dietary fibre method was evaluated and accepted by Accepted: March 5, 2015 AOAC InternationalandAACCInternational(AOACMethods2009.01and2011.25;AACCMethod 32–45.01 and 32–50.01, and recently adopted by Codex Alimentarius as a Type I Method. However, in application of the method to a diverse range of food samples and particularly food ingredients, some limitations have been identified. One of the ongoing criticisms of this method was that the time of incubation with pancreatic a-amylase/amyloglucosidase mixture was 16 h, whereas the time for food to transit through the human small intestine was likely to be approximately 4 h. In the current work, we use an incubation time of 4 h, and have evaluated incubation conditions that yield resistant starch and dietary values in line with ileostomy results within this time frame. Problems associated with production, hydrolysis and chromatography of various oligosaccharides have been addressed resulting in a more rapid procedure that is directly applicable to all foods and food ingredients currently available. Keywords: Available carbohydrates / Codex Alimentarius / Dietary fibre determination / Enzymic / Non-digestible oligosaccharides / Resistant starch : Additional supporting information may be found in the online version of this article at the publisher’s web-site. -

BASICS of the FODMAP DIET Elizabeth English RD, CLC OBJECTIVES
BASICS OF THE FODMAP DIET Elizabeth English RD, CLC OBJECTIVES Describe sources of FODMAP carbohydrate Recognize appropriate patient populations for the FODMAP diet Identify high FODMAP foods which are necessary to restrict when following the FODMAP diet Identify low FODMAP foods which are allowed when following the FODMAP diet FODMAP • Fermentable • Oligosaccharides • Disaccharides • Monosaccharides • And • Polyols POPULATION • Prevalence of IBS varies between 8% - 20% of the US population depending on diagnostic criteria and population evaluated • Most studies report a higher prevalence of IBS in women than men • Average medical expenditure for IBS in the US is estimated to be $1.35 billion in direct costs and $205 million in indirect costs • IBS accounts for almost half of all visits to gastroenterologists MAGGE & LEMBO . GASTORENTEROLOGY & HEPATOLOGY 2012 KIDS • Kids with FGID (functional gastrointestinal disorders) report lower quality of life than healthy controls •Increased incidence of school absenteeism •Decreased energy •Less likely to be physically active •Less likely to be involved in school activities •Increased feelings of sadness and loneliness YOUSSEF ET AL. PEDIATRICS 2014 TARGET POPULATION • Patients diagnosed with functional gastrointestinal diseases (FGIDs) including IBS, abdominal migrane & childhood functional abdominal pain • Diagnosis by exclusion • Celiac disease • IBD • Food allergies • EoE • Cancer • Gastritis FODMAP HISTORY • Sue Shepherd developed the FODMAP diet in 1999 at her Shepherd Works RD practice • Realized that FODMAP foods were triggers for IBS • In 2005, the first paper describing FODMAPs was published with Dr. Peter Gibson • In 2006, the first research trial was a retrospective audit of patients with IBS and fructose malabsorption on a low fructose/fructan diet with 74% of patients reporting symptomatic improvement on this dietary regimen • Early research continued until 2009 when the FODMAP diet became well known throughout the digestive community BARRETT & GIBSON. -

48 3-1-4. Reactions Using Mutant FSA A129S Another Mutant Enzyme
Results _ 3-1-4. Reactions using mutant FSA A129S Another mutant enzyme, A129S, which has serine at 129th residue instead of alanine (Fig.3-11a), has high potential to catalyze the reactions using DHA as a donor substrate. Virtually, the kcat/Km value for DHA is more than 12 fold of that of WT (Schürmann, 2001). This implies that larger amounts of products can be acquired with A129S than with WT, or unfavorable reactions with WT may be accomplished with the mutant enzyme. Firstly, FSA A129S was produced in DH5α with pUC18 plasmid vector and purified with same method as WT was done (Fig.3-11b, Table 3-8). This mutant enzyme was overexpressed well and a strong band corresponding to FSA was seen on SDS-PAGE gel (as 10~15% of total protein in crude extract). In addition, this mutant enzyme held the stability against high temperature. Thus, a heat treatment step was part of the purification protocol. However, protein solution obtained from hydroxyapatite column of the final purification step showed still a few extra protein bands on SDS-PAGE gel (Fig.3-11b). Although it contained those extra proteins, the purity appeared to be more than 90% on the gel and the specific activity was quite high enough to assay the catalytic ability of the enzyme for several substrates. Indeed, even crude extract showed higher specific activity than purified WT had (Table 3-8). Three reactions (scheme 3-1) were probed to assay catalytic ability of both FSA WT and A129S. Dihydroxyacetone (DHA) or hydroxyacetone (HA) were used as donor substrates, and formaldehyde or glycolaldehyde as acceptor. -

Pharmaceutical Compositions of Rifaximin Pharmazeutische Rifaximin-Zusammensetzungen Compositions Pharmaceutiques De Rifaximine
(19) TZZ Z__ T (11) EP 2 011 486 B2 (12) NEW EUROPEAN PATENT SPECIFICATION After opposition procedure (45) Date of publication and mention (51) Int Cl.: of the opposition decision: A61K 9/20 (2006.01) A61K 31/44 (2006.01) 12.08.2015 Bulletin 2015/33 (45) Mention of the grant of the patent: 23.05.2012 Bulletin 2012/21 (21) Application number: 08252198.0 (22) Date of filing: 26.06.2008 (54) Pharmaceutical compositions of rifaximin Pharmazeutische Rifaximin-Zusammensetzungen Compositions pharmaceutiques de rifaximine (84) Designated Contracting States: (56) References cited: AT BE BG CH CY CZ DE DK EE ES FI FR GB GR EP-A1- 0 616 808 EP-B1- 1 763 339 HR HU IE IS IT LI LT LU LV MC MT NL NO PL PT WO-A-2006/094737 WO-A2-2006/039022 RO SE SI SK TR US-A- 6 140 355 US-A1- 2005 101 598 (30) Priority: 06.07.2007 IN KO09682007 • DUPONT ET AL: "Treatment of Travelers’ 23.06.2008 EP 08252158 Diarrhea: Randomized Trial Comparing Rifaximin, Rifaximin Plus Loperamide, and (43) Date of publication of application: Loperamide Alone" CLINICAL 07.01.2009 Bulletin 2009/02 GASTROENTEROLOGY AND HEPATOLOGY, AMERICAN GASTROENTEROLOGICAL (60) Divisional application: ASSOCIATION, US, vol. 5, no. 4, 17 April 2007 11176043.5 / 2 420 226 (2007-04-17), pages 451-456, XP022029177 ISSN: 14186563.4 / 2 837 378 1542-3565 • ARYA ET AL: "Rifaximin-the promising anti- (73) Proprietor: Lupin Ltd. microbial for enteric infections" JOURNAL OF Mumbai, Maharashtra 400 098 (IN) INFECTION, ACADEMIC PRESS, LONDON, GB, vol. -

Biochemistry Entry of Fructose and Galactose
Paper : 04 Metabolism of carbohydrates Module : 06 Entry of Fructose and Galactose Dr. Vijaya Khader Dr. MC Varadaraj Principal Investigator Dr.S.K.Khare,Professor IIT Delhi. Paper Coordinator Dr. Ramesh Kothari,Professor UGC-CAS Department of Biosciences Saurashtra University, Rajkot-5, Gujarat-INDIA Dr. S. P. Singh, Professor Content Reviewer UGC-CAS Department of Biosciences Saurashtra University, Rajkot-5, Gujarat-INDIA Dr. Charmy Kothari, Assistant Professor Content Writer Department of Biotechnology Christ College, Affiliated to Saurashtra University, Rajkot-5, Gujarat-INDIA 1 Metabolism of Carbohydrates Biochemistry Entry of Fructose and Galactose Description of Module Subject Name Biochemistry Paper Name 04 Metabolism of Carbohydrates Module Name/Title 06 Entry of Fructose and Galactose 2 Metabolism of Carbohydrates Biochemistry Entry of Fructose and Galactose METABOLISM OF FRUCTOSE Objectives 1. To study the major pathway of fructose metabolism 2. To study specialized pathways of fructose metabolism 3. To study metabolism of galactose 4. To study disorders of galactose metabolism 3 Metabolism of Carbohydrates Biochemistry Entry of Fructose and Galactose Introduction Sucrose disaccharide contains glucose and fructose as monomers. Sucrose can be utilized as a major source of energy. Sucrose includes sugar beets, sugar cane, sorghum, maple sugar pineapple, ripe fruits and honey Corn syrup is recognized as high fructose corn syrup which gives the impression that it is very rich in fructose content but the difference between the fructose content in sucrose and high fructose corn syrup is only 5-10%. HFCS is rich in fructose because the sucrose extracted from the corn syrup is treated with the enzyme that converts some glucose in fructose which makes it more sweet. -

United States Patent Office
- 2,926,180 United States Patent Office Patented Feb. 23, 1960 2 cycloalkyl, etc. These substituents R and R' may also be substituted with various groupings such as carboxyl 2,926,180 groups, sulfo groups, halogen atoms, etc. Examples of CONDENSATION OF AROMATIC KETONES WITH compounds which are included within the scope of this CARBOHYDRATES AND RELATED MATER ALS 5 general formula are acetophenone, propiophenone, benzo Carl B. Linn, Riverside, Ill., assignor, by mesne assign phenone, acetomesitylene, phenylglyoxal, benzylaceto ments, to Universal Oil Products Company, Des phenone, dypnone, dibenzoylmethane, benzopinacolone, Plaines, Ill., a corporation of Delaware dimethylaminobenzophenone, acetonaphthalene, benzoyl No Drawing. Application June 18, 1957 naphthalene, acetonaphthacene, benzoylnaphthacene, ben 10 zil, benzilacetophenone, ortho-hydroxyacetophenone, para Serial No. 666,489 hydroxyacetophenone, ortho - hydroxy-para - methoxy 5 Claims. (C. 260-345.9) acetophenone, para-hydroxy-meta-methoxyacetophenone, zingerone, etc. This application is a continuation-in-part of my co Carbohydrates which are condensed with aromatic pending application Serial No. 401,068, filed December 5 ketones to form a compound selected from the group 29, 1953, now Patent No. 2,798,079. consisting of an acylaryl-desoxy-alditol and an acylaryl This invention relates to a process for interacting aro desoxy-ketitol include simple sugars, their desoxy- and matic ketones with carbohydrates and materials closely omega-carboxy derivatives, compound sugars or oligo related to carbohydrates. The process relates more par saccharides, and polysaccharides. ticularly to the condensation of simple sugars, their 20 Simple sugars include dioses, trioses, tetroses, pentoses, desoxy- and their omega-carboxy derivatives, compound hexoses, heptoses, octoses, nonoses, and decoses. Com sugars or oligosaccharides, and polysaccharides with aro pound sugars include disaccharides, trisaccharides, and matic ketones in the presence of a hydrogen fluoride tetrasaccharides. -

Sia: the Sugar Code of Life
Journal of General Surgery Open Access Full Text Article Research Article Sia: The Sugar Code of Life This article was published in the following Scient Open Access Journal: Journal of General Surgery Received November 05, 2017; Accepted November 27, 2017; Published December 04, 2017 Robert Skopec* Abstract Analyst-researcher, Dubnik, Slovakia, Europe Our cells are coated with sugar, and when it comes to cancer, that’s anything but sweet. In a recent talk at TEDxStanford, chemist Carolyn Bertozzi explained why. She studies sialic acid, a sugar that seems to deceive the immune system, allowing cancer cells to evade the body’s defenses. This work focuses on the complex, sugary structures surrounding human cells. That foliage-like coating, it turns out, can tell us a lot of our body – it even reveals a patient’s blood type. Sugar and carbohydrates are dangerous supporters of different types of cancer. Introduction As it can be seen from the information of the American Chemical Society (ACS), ideally, the immune system can figure out which cells are bad, attack them and protect the body from disease. In the case of cancer cells, though, a special sugary coating tricks the immune system into ignoring them. The sialic acid, a sugar that’s denser in cancer cells than in other cells. It seems deceive the immune system, allowing the cancer cells to evade the body’s defenses. Unnoticed and unchallenged, cancer cells are free to divideMonosaccharides and run wild inside - Sialic the acid/N-acetylneuraminicbody [1,2]. acid N-acetylneuraminic acid, also known as Sialic acid, is a key component of important amino sugars that mediate cellular communication. -
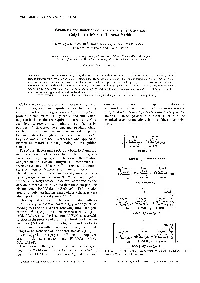
Synthesis and Functions of a Glycopolymer Carrying Gal/Jl-+4(Glcnach Tetrasaccharide
Polymer Journal, Vol. 30. No. 8, pp 653-658 ( 1998) Synthesis and Functions of a Glycopolymer Carrying Gal/Jl-+4(GlcNAch Tetrasaccharide Kazukiyo KoBAYASHI,t Shoko KAMIYA, Minoru MATSUYAMA,* Takeomi MURATA,* and Taichi Usur* Graduate School of' Engineering, Nagoya University, Chikusa, Nagoya 464-8603, Japan * Faculty of' Agriculture, Shizuoka University, Ohya, Shizuoka 422---8529, Japan (Received February 16, 1998) ABSTRACT: Tetrasaccharide Galfil--+4(GlcNAch was synthesized from N,N',N"-triacetylchitotriose (GlcNAc)s and lactose using transglycosylation with a P-o-galactosidase from Bacillus circu/ans. The reducing terminal of Galfil--+4(GlcNAclJ was oxidized and connected to p-vinylbenzylamine via amide linkage, and the resulting oligosaccharide-substituted styrene monomer was polymerized with the radical initiator, 2,2' -azobis(2-amidinopropane) dihydrochloride at 60"C. Glycopolystyrene was found to bind strongly with wheat germ agglutinin (WGA) and tomato (Lycopersicon esculentum) agglutinin (LEA) by inhibition of hemagglutination and double diffusion. KEY WORDS Oligosaccharides / Glycopolymers / Lectins / Enzymatic Synthesis/ Recognition / Cell surface carbohydrates from glycoproteins, glyco dimensional immunodiffusion in agar and inhibition of lipids, proteoglycans, and capsular polysaccharides play lectin-induced hemagglutination. Comparison was made important roles in biological events, 1.2 Carbohydrate using analogous homopolymers carrying N-acetyllactos protein interaction is usually weak and multivalent amine and chitooligosaccharides. Chart I illustrates the oligosaccharide chains are required to target cell surface chemical structures and abbreviations of these glycopoly carbohydrate receptors and inhibit host infection by mers. pathogens. 3 Glycopolymers carrying pendant oligo saccharide chains can be regarded as multivalent ligands known to interact strongly with lectins and antibodies, 4 · 5 Glycopolymers have been synthesized and applied as biomedical materials having biological recognition signals. -

Assessment of DHA in Self-Tanning Creams Applied in Spray Booths
Assessment of DHA in self-tanning creams applied in spray booths Lena Höglund MSc. DTC (Danish Toxicology Centre): Betty Bügel Mogensen Rossana Bossi Marianne Glasius National Environmental Research Institute of Denmark Survey of Chemical Substances in Consumer Products, No. 72 2006 The Danish Environmental Protection Agency will, when opportunity offers, publish reports and contributions relating to environmental research and development projects financed via the Danish EPA. Please note that publication does not signify that the contents of the reports necessarily reflect the views of the Danish EPA. The reports are, however, published because the Danish EPA finds that the studies represent a valuable contribution to the debate on environmental policy in Denmark. Contents SUMMARY AND CONCLUSIONS 5 1 INTRODUCTION 7 2 OBJECTIVES 10 3 TECHNIQUES 12 3.1 DESCRIPTION OF TECHNIQUES 12 3.1.1 Manual turbine spray 12 3.1.2 Third-generation booths (closed booths) 13 3.1.3 Fourth-generation booths (open booths) 15 3.2 SAFETY INSTRUCTIONS 16 3.2.1 General remarks on enterprises' safety instructions 16 3.2.2 Safety instructions from the authorities 16 3.2.3 Advice for customers from personnel 16 4 SUBSTANCES CONTAINED IN PRODUCTS 20 5 HEALTH ASSESSMENT 22 5.1 TOXOCOLOGICAL PROFILE OF DIHYDROXYACETONE (DHA) (CAS NO. 96-26-4) 22 5.2 BRIEF HEALTH ASSESSMENT OF ETHOXYDIGLYCOL (CAS NO. 111-90-0) 26 5.3 BRIEF HEALTH ASSESSMENT OF PHENOXYETHANOL (CAS NO.122-99-6) 27 5.4 BRIEF HEALTH ASSESSMENT OF GLYCERINE (CAS NO. 56-81-5) 27 5.5 BRIEF HEALTH ASSESSMENT OF POLYSORBATES AND SORBITAN ESTERS 27 5.6 BRIEF HEALTH ASSESSMENT OF ERYTHRULOSE (CAS NO. -

Unit 1: Carbohydrates Structure and Biological Importance: Monosaccharides, Disaccharides, Polysaccharides and Glycoconjugates
CBCS 3rd Semester Core Course VII Paper: Fundamentals of Biochemistry Unit 1: Carbohydrates Structure and Biological importance: Monosaccharides, Disaccharides, Polysaccharides and Glycoconjugates By- Dr. Luna Phukan Definition The carbohydrates are a group of naturally occurring carbonyl compounds (aldehydes or ketones) that also contain several hydroxyl groups. It may also include their derivatives which produce such compounds on hydrolysis. They are the most abundant organic molecules in nature and also referred to as “saccharides”. The carbohydrates which are soluble in water and sweet in taste are called as “sugars”. Structure of Carbohydrates Carbohydrates consist of carbon, hydrogen, and oxygen. The general empirical structure for carbohydrates is (CH2O)n. They are organic compounds organized in the form of aldehydes or ketones with multiple hydroxyl groups coming off the carbon chain. The building blocks of all carbohydrates are simple sugars called monosaccharides. A monosaccharide can be a polyhydroxy aldehyde (aldose) or a polyhydroxy ketone (ketose) The carbohydrates can be structurally represented in any of the three forms: 1. Open chain structure. 2. Hemi-acetal structure. 3. Haworth structure. Open chain structure – It is the long straight-chain form of carbohydrates. Hemi-acetal structure – Here the 1st carbon of the glucose condenses with the -OH group of the 5th carbon to form a ring structure. Haworth structure – It is the presence of the pyranose ring structure Classification of Carbohydrates Monosaccharides The simple carbohydrates include single sugars (monosaccharides) and polymers, oligosaccharides, and polysaccharides. Simplest group of carbohydrates and often called simple sugars since they cannot be further hydrolyzed. Colorless, crystalline solid which are soluble in water and insoluble in a non-polar solvent. -

PENTOSE PHOSPHATE PATHWAY — Restricted for Students Enrolled in MCB102, UC Berkeley, Spring 2008 ONLY
Metabolism Lecture 5 — PENTOSE PHOSPHATE PATHWAY — Restricted for students enrolled in MCB102, UC Berkeley, Spring 2008 ONLY Bryan Krantz: University of California, Berkeley MCB 102, Spring 2008, Metabolism Lecture 5 Reading: Ch. 14 of Principles of Biochemistry, “Glycolysis, Gluconeogenesis, & Pentose Phosphate Pathway.” PENTOSE PHOSPHATE PATHWAY This pathway produces ribose from glucose, and it also generates 2 NADPH. Two Phases: [1] Oxidative Phase & [2] Non-oxidative Phase + + Glucose 6-Phosphate + 2 NADP + H2O Ribose 5-Phosphate + 2 NADPH + CO2 + 2H ● What are pentoses? Why do we need them? ◦ DNA & RNA ◦ Cofactors in enzymes ● Where do we get them? Diet and from glucose (and other sugars) via the Pentose Phosphate Pathway. ● Is the Pentose Phosphate Pathway just about making ribose sugars from glucose? (1) Important for biosynthetic pathways using NADPH, and (2) a high cytosolic reducing potential from NADPH is sometimes required to advert oxidative damage by radicals, e.g., ● - ● O2 and H—O Metabolism Lecture 5 — PENTOSE PHOSPHATE PATHWAY — Restricted for students enrolled in MCB102, UC Berkeley, Spring 2008 ONLY Two Phases of the Pentose Pathway Metabolism Lecture 5 — PENTOSE PHOSPHATE PATHWAY — Restricted for students enrolled in MCB102, UC Berkeley, Spring 2008 ONLY NADPH vs. NADH Metabolism Lecture 5 — PENTOSE PHOSPHATE PATHWAY — Restricted for students enrolled in MCB102, UC Berkeley, Spring 2008 ONLY Oxidative Phase: Glucose-6-P Ribose-5-P Glucose 6-phosphate dehydrogenase. First enzymatic step in oxidative phase, converting NADP+ to NADPH. Glucose 6-phosphate + NADP+ 6-Phosphoglucono-δ-lactone + NADPH + H+ Mechanism. Oxidation reaction of C1 position. Hydride transfer to the NADP+, forming a lactone, which is an intra-molecular ester.