Observing Imperfection in Atomic Interfaces for Van Der Waals
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

NEWSLETTER January 2020
NEWSLETTER January 2020 ADF-STEM image recorded using a JEOL ARM200 double corrected microscope along the <110> direction of GaAsP. The image reveals the presence of defects at the tip of GaAsP nanowires. Scale bar: 2 nm (image courtesy of James Gott, University of Warwick) See http://emag.iop.org for further details EMAG Group newsletter Jan 2020 CONTENTS EMAG COMMITTEE 4 LETTER FROM THE CHAIR 5 FORTHCOMING EVENTS 6 EMAG 2020: 6 MICROSCOPY ENABLED BY DIRECT ELECTRON DETECTION 6 HYPERSPY WORKSHOP 7 SUPERSTEM SUMMER SCHOOL, 7 EMAG ANNUAL GENERAL MEETING 2020 7 NEWS 8 SUPERSTEM FACILITY UPDATES 8 QUEEN’S UNIVERSITY BELFAST JOINS THE GROUP OF CLIMATE IN-SITU USERS 9 RESEARCH HIGHLIGHTS BY YOUNG SCIENTISTS 10 THE EFFECTS OF CORROSION ON SILVER NANOPARTICLE PLASMONICS 10 MACHINE LEARNING FOR NANOPARTICLE DISPERSION ANALYSIS IN COMPLEX MEDIA 12 ELECTRON PTYCHOGRAPHY USING FAST BINARY 4D STEM DATA 13 MEETING REPORTS 15 MMC2019/EMAG, MANCHESTER 15 MC2019, BERLIN 16 MICROSCOPY INFRASTRUCTURES ACCESS SCHEME 17 SUPERSTEM 17 EPSIC 17 ESTEEM 3 18 LEEDS EPSRC NANOSCIENCE AND NANOTECHNOLOGY FACILITY (LENNF) 18 SIR HENRY ROYCE INSTITUTE 20 APPLY FOR IOP RESEARCH STUDENTS CONFERENCE FUND 21 2 EMAG Group newsletter Jan 2020 BURSARIES REPORTS (2019) 21 EMAS MEETING MAY 2019 21 EMS MEMBERSHIP 22 ADDITIONAL FUTURE MEETINGS OF INTEREST 23 26th Australian Conference on Microscopy and Microanalysis 23 15th European Molecular Imaging Meeting – EMIM 2020 23 EBSD 2020 23 Electron Microscopy Summer School 2020 23 8th International Workshop on Focused Electron Beam-Induced -

A Working Group on the Provision of Electron Microscopy in the Physical and Engineering Sciences (EMPESWG)
1 A working group on the provision of electron microscopy in the physical and engineering sciences (EMPESWG) Final report Executive summary This report describes the outcomes of a process that aimed to review the current ecosystem for electron microscopy (EM) in the physical and engineering sciences, to project future needs, and to look at ways of improving coordination within the EM community to maximise the cost-effectiveness of the sector. The process started with a Town Hall meeting in 2009, followed by a more detailed study by a working group (WG), the outcomes of which were presented to the community in a further Town Hall meeting in April 2014, and feedback from which was incorporated into the writing of this final report from the WG. The WG identified 4 key areas to focus on: (i) evaluation of the current ecosystem; (ii) coordination of access; (iii) coordination of training and (iv) identification of future technologies and projection of future needs. Each area led to recommendations summarised here: (i) The Current Ecosystem This part of the report made use of evidence from surveys conducted of both EM laboratory leaders and also of users of EM facilities. The recommendations are: • Enlarge the survey base (both for laboratory leaders and particularly the large breadth of EM users) and re-evaluate the current ecosystem at regular intervals; • Set up a database for electron microscopy and related equipment, and staff expertise; • Review the performance of previous EM user access schemes and make recommendations of best practice for future access schemes, for the benefit of the UK EM community. -

Improved Filtering of Electron Tomography EDX Data
University of South Carolina Scholar Commons Senior Theses Honors College Spring 2020 Improved Filtering of Electron Tomography EDX Data Kelsey M. Larkin University of South Carolina - Columbia, [email protected] Follow this and additional works at: https://scholarcommons.sc.edu/senior_theses Part of the Numerical Analysis and Computation Commons Recommended Citation Larkin, Kelsey M., "Improved Filtering of Electron Tomography EDX Data" (2020). Senior Theses. 351. https://scholarcommons.sc.edu/senior_theses/351 This Thesis is brought to you by the Honors College at Scholar Commons. It has been accepted for inclusion in Senior Theses by an authorized administrator of Scholar Commons. For more information, please contact [email protected]. Improved Filtering of Electron Tomography EDX Data By Kelsey Mary Larkin Submitted in Partial Fulfillment of the Requirements for Graduation with Honors from the South Carolina Honors College May 2020 Approved: Peter Binev Major Professor Thomas Vogt Second Reader Steve Lynn, Dean For South Carolina Honors College c Copyright by Kelsey Mary Larkin, 2020 All Rights Reserved. ii Dedication This thesis is dedicated to my support system of professors, family, and friends who have encouraged me through my educational endeavors at the University of South Carolina. Without their love and support I would have never been able to accomplish my goals. iii Acknowledgments This thesis would not have been possible without the help and support of many people. Dr. Peter Binev has been the most incredible mentor and supporter. The field of data processing is expansive and complex, but he was devoted to my understanding of the material and his patience was endless. -

PDF Reprint of Publication
Article www.acsnano.org Atomic Defects and Doping of Monolayer NbSe2 † ‡ § ∥ ∥ Lan Nguyen, Hannu-Pekka Komsa, Ekaterina Khestanova, Reza J. Kashtiban, Jonathan J. P. Peters, † ∥ ∥ § § Sean Lawlor, Ana M. Sanchez, Jeremy Sloan, Roman V. Gorbachev, Irina V. Grigorieva, ⊥ # ¶ † Arkady V. Krasheninnikov, , , and Sarah J. Haigh*, † § School of Materials and School of Physics and Astronomy, University of Manchester, Oxford Road, Manchester, M13 9PL, United Kingdom ‡ ¶ COMP Centre of Excellence, Department of Applied Physics, and Department of Applied Physics, Aalto University, P.O. Box 11100, FI-00076 Aalto, Finland ∥ Department of Physics, University of Warwick, Coventry, CV4 7AL, United Kingdom ⊥ Institute of Ion Beam Physics and Materials Research, Helmholtz-Zentrum Dresden-Rossendorf, 01328 Dresden, Germany # National University of Science and Technology MISiS, Leninskiy Prospekt, Moscow, 119049, Russian Federation *S Supporting Information ABSTRACT: We have investigated the structure of atomic defects within monolayer NbSe2 encapsulated in graphene by combining atomic resolution transmission electron microscope imaging, density functional theory (DFT) calculations, and strain mapping using geometric phase analysis. We demonstrate the presence of stable Nb and Se monovacancies in monolayer material and reveal that Se monovacancies are the most frequently observed defects, consistent with DFT calculations of their formation energy. We reveal that adventitious impurities of C, N, and O can substitute into the NbSe2 lattice stabilizing Se divacancies. We further observe evidence of Pt substitution into both Se and Nb vacancy sites. This knowledge of the character and relative frequency of different atomic defects provides the potential to better understand and control the unusual electronic and magnetic properties of this exciting two-dimensional material. -

Work-Stealing Prefix Scan: Addressing Load Imbalance in Large
1 Work-stealing prefix scan: Addressing load imbalance in large-scale image registration Marcin Copik1, Tobias Grosser2, Torsten Hoefler1, Member, IEEE, Paolo Bientinesi3, Benjamin Berkels4 1Department of Computer Science, ETH Zurich; 2School of Informatics, University of Edinburgh; 3Department of Computing Science, Umea˚ University; 4AICES, RWTH Aachen University Abstract—Parallelism patterns (e.g., map or reduce) have proven to be effective tools for parallelizing high-performance applications. In this paper, we study the recursive registration of a series of electron microscopy images – a time consuming and imbalanced computation necessary for nano-scale microscopy analysis. We show that by translating the image registration into a specific instance of the prefix scan, we can convert this seemingly sequential problem into a parallel computation that scales to over thousand of cores. We analyze a variety of scan algorithms that behave similarly for common low-compute operators and propose a novel work-stealing procedure for a hierarchical prefix scan. Our evaluation shows that by identifying a suitable and well-optimized prefix scan algorithm, we reduce time-to-solution on a series of 4,096 images spanning ten seconds of microscopy acquisition from over 10 hours to less than 3 minutes (using 1024 Intel Haswell cores), enabling derivation of material properties at nanoscale for long microscopy image series. F 1 INTRODUCTION 250 Many seemingly sequential algorithms in which the Ideal Speedup computation of element xi+1 depends on element xi can be Distributed, Dissemination parallelized with a prefix scan operation. Such an operation Distributed, Ladner-Fischer 200 Distributed, MPI_Scan takes a binary and associative operator and an input array Work-stealing, Dissemination x0; x1; : : : ; xn and produces the output array y0; y1; : : : ; yn. -

Stream 1: EMAG - 2D Materials 10:00 - 12:00 Tuesday, 6Th July, 2021 Sessions EMAG Conference Session Session Organiser Andy Brown, Sarah Haigh
Stream 1: EMAG - 2D Materials 10:00 - 12:00 Tuesday, 6th July, 2021 Sessions EMAG Conference Session Session Organiser Andy Brown, Sarah Haigh 10:00 - 10:30 349 Atomic Imaging in 2D Material Heterostructures : Twist, Defects and Particle Synthesis Prof Sarah Haigh1, Dr Nick Clark1, Astrid Weston1, Dr Daniel Kelly1, Dr Matthew Hamer1, Dr Yichao Zou1, Dr Vladimir Enaldiev1, Dr Alex Summerfield1, Dr Victor Zólyomi1, Prof Denis Gebauer2, Prof Vladimir Falko1, Dr Roman Gorbachev1 1National Graphene Institute, University of Manchester, Manchester, United Kingdom. 2Institute of Inorganic Chemistry, Leibniz Universität Hannover, Hannover, Germany Abstract Text This talk aims to demonstrate how atomic resolution scanning transmission electron microscope (STEM) imaging is being used in Manchester to support and enable the development of 2D materials and their heterostructures. The possibility to create new ‘designer’ materials by stacking together atomically thin layers extracted from layered materials with different properties has opened up a huge range of opportunities, from new optoelectronic phenomena [1], modifying and enhancing electron interactions in moiré superlattices [2], to creating a totally new concept of designer nanochannels for molecular or ionic transport [3]. The impressive progress being achieved in the field crucially depends on knowledge of the atomic structure of these heterostructures [4], which in many cases can only be analysed by transmission electron microscopy (TEM) techniques. In this talk I will try to illustrate this with some of our recent work. I will demonstrate imaging of the unusual lattice reconstruction that occurs in twisted transition metal dicholcogenide bilayers [5]. We reveal that this behaviour is more complex than is seen for twisted heterostructures of graphene and/or hexagonal boron nitride. -

Atomic Resolution Imaging of Crbr3 Using Adhesion-Enhanced Grids
Atomic Resolution Imaging of CrBr3 using Adhesion-Enhanced Grids Matthew J. Hamer1,2, David G. Hopkinson2,3, Nick Clark2,3, Mingwei Zhou1,2, Wendong Wang1,2 Yichao Zou3, Daniel J. Kelly2,3, Thomas H. Bointon2, Sarah J. Haigh2,3,**, Roman V. Gorbachev1,2,4,* 1Department of Physics and Astronomy, University of Manchester, Oxford Road, Manchester, M13 9PL, UK 2National Graphene Institute, University of Manchester, Oxford Road, Manchester, M13 9PL, UK 3Department of Materials, University of Manchester, Oxford Road, Manchester, M13 9PL, UK 4Henry Royce Institute, Oxford Road, Manchester, M13 9PL, UK *[email protected], **[email protected] KEYWORDS: Magnetic 2D Materials, TEM, Graphene Encapsulation, Crystal Transfer, Suspended Devices Abstract Suspended specimens of 2D crystals and their heterostructures are required for a range of studies including transmission electron microscopy (TEM), optical transmission experiments and nanomechanical testing. However, investigating the properties of laterally small 2D crystal specimens, including twisted bilayers and air sensitive materials, has been held back by the difficulty of fabricating the necessary clean suspended samples. Here we present a scalable solution which allows clean free-standing specimens to be realized with 100% yield by dry- stamping atomically thin 2D stacks onto a specially developed adhesion-enhanced support grid. Using this new capability, we demonstrate atomic resolution imaging of defect structures in atomically thin CrBr3, a novel magnetic material which degrades in ambient conditions. Introduction The family of 2D materials now includes over a hundred members, many of which have been isolated in monolayer form by mechanical exfoliation1,2, direct chemical growth3,4 or solution- phase processing5. -
Autocorrected Off-Axis Holography of 2D Materials
Autocorrected Off-axis Holography of 2D Materials Felix Kern,1 Martin Linck,2 Daniel Wolf,1 Nasim Alem,3 Himani Arora,4 Sibylle Gemming,4 Artur Erbe,4 Alex Zettl,5 Bernd Büchner,1 and Axel Lubk1 1Institute for Solid State Research, IFW Dresden, Helmholtzstr. 20, 01069 Dresden, Germany 2Corrected Electron Optical Systems GmbH, Englerstr. 28, D-69126 Heidelberg, Germany 3Department of Materials Science and Engineering, Pennsylvania State University, N-210 Millennium Science Complex University Park, PA 16802, United States 4Helmholtz-Zentrum Dresden-Rossendorf, Dresden 01328, Germany 5Department of Physics, University of California Berkeley, 366 LeConte Hall MC 7300 Berkeley, CA, California 94720-7300, USA The reduced dimensionality in two-dimensional materials leads a wealth of unusual properties, which are currently explored for both fundamental and applied sciences. In order to study the crystal structure, edge states, the formation of defects and grain boundaries, or the impact of adsorbates, high resolution microscopy techniques are indispensible. Here we report on the development of an electron holography (EH) transmission electron microscopy (TEM) technique, which facilitates high spatial resolution by an automatic correction of geometric aberrations. Distinguished features of EH beyond conventional TEM imaging are the gap-free spatial information signal transfer and higher dose efficiency for certain spatial frequency bands as well as direct access to the projected electrostatic potential of the 2D material. We demonstrate these features at the example of h-BN, at which we measure the electrostatic potential as a function of layer number down to the monolayer limit and obtain evidence for a systematic increase of the potential at the zig-zag edges. -

Graphene Flakes Present in Either a Liquid Dispersion Or Powder Form
The National Physical Laboratory (NPL) NPL is the UK’s National Measurement Institute, and is a world-leading centre of excellence in developing and applying the most accurate measurement standards, science and technology available. NPL's mission is to provide the measurement capability that underpins the UK's prosperity and quality of life. © NPL Management Limited, 2017 Version 1.0 NPL Authors and Contributors Dr Andrew J Pollard Dr Keith R. Paton Dr Charles A. Clifford Ms Elizabeth Legge Find out more about NPL measurement training at www.npl.co.uk/training or our e-learning Training Programme at www.npl.co.uk/e-learning National Physical Laboratory Telephone: +44 (0)20 8977 3222 Hampton Road e-mail: [email protected] Teddington www.npl.co.uk Middlesex TW11 0LW United Kingdom 2 The National Graphene Institute (NGI) at the University of Manchester The £61m National Graphene Institute (NGI) is the world’s leading centre of graphene research and commercialisation. Opened in March 2015, it is not only home to graphene scientists from The University of Manchester but also from across the UK, partnering with leading commercial organisations interested in producing the applications of the future. Currently the NGI has more than 40 industrial partners, working on a range of applications across a wide variety of disciplines. With 7,800 square metres of collaborative research facilities and 1,500 square metres of cleanroom space, the NGI is the largest academic space of its kind in the world for dedicated graphene research. The NGI is funded by £38m from the Engineering and Physical Sciences Research Council (EPSRC) and £23m from the European Regional Development Fund. -

Seminaires Ipcms
INSTITUT DE PHYSIQUE ET CHIMIE DES MATERIAUX DE STRASBOURG 23, rue du Loess – BP 43 67034 STRASBOURG CEDEX 02 03 88 10 71 41 SEMINAIRE IPCMS Vendredi 20 mars 2020 à 15h à l’auditorium « New challenges for scanning transmission electron microscope imaging of nanomaterials : 2D material heterostructures, liquid cells and 3D nanoparticle catalytic chemistry » Prof. Sarah HAIGH Director of the Electron Microscopy Centre Deputy Director of BP ICAM University of Manchester, Department of Materials Contact : Pierre Rabu ([email protected]) Abstract: The properties of nanomaterials are known to be sensitive to their size, shape and elemental distribution. Scanning transmission electron microscopy (STEM) is a powerful tool for investigating the local structure and chemistry of a wide range of different nanomaterials but the technique still has a number of limitations. The first problem is that specimens are usually examined in the microscope’s high vacuum environment. Examining materials under more realistic environmental conditions is highly desirable but usually requires us to sacrifice spatial resolution or elemental analysis capabilities. In this talk I will describe some recent advances in development of in situ analytical STEM including our use of graphene as a perfect electron transparent window [1]. The other common problem with STEM is the potential for damage caused by the electron beam. I will describe a new approach where we have learnt from the Nobel prize winning technique of single particle reconstruction, often applied to biological systems, to yield 3D elemental information for beam sensitive inorganic nanoparticles [2]. Finally I will demonstrate an approach for gaining 3D structural information to investigate local reconstruction in twisted 2D material heterostructures [3]. -
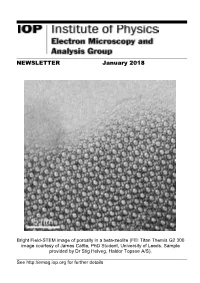
NEWSLETTER January 2018
NEWSLETTER January 2018 Bright Field-STEM image of porosity in a beta-zeolite (FEI Titan Themis G2 300 image courtesy of James Cattle, PhD Student, University of Leeds. Sample provided by Dr Stig Helveg, Haldor Topsoe A/S). See http://emag.iop.org for further details EMAG Group newsletter Jan 2018 CONTENTS EMAG COMMITTEE 3 LETTER FROM THE CHAIR 4 FORTHCOMING EVENTS 5 EMAG 2018 CALL FOR PAPERS 5 EMAG ANNUAL GENERAL MEETING 2018 6 NEWS 7 PROF. PRATIBHA GAI DBE 7 UNIVERSITY OF GLASGOW PLASMA-FIB 8 ELECTRON MICROSCOPY AT DARESBURY LABS 10 MEETING REPORTS 11 MSM-XX, OXFORD 11 VISION AND OPHTHALMOLOGY, BALTIMORE 12 MMC17, MANCHESTER 14 M&M, ST LOUIS 15 APPLY FOR IOP RESEARCH STUDENTS CONFERENCE FUND 15 EMS MEMBERSHIP 16 ADDITIONAL FUTURE MEETINGS OF INTEREST 17 2 EMAG Group newsletter Jan 2018 EMAG COMMITTEE Chair Dr Sarah Haigh School of Materials, University of Manchester, Manchester, M13 9PL Tel: 0161 306 3618 Email: [email protected] Secretary & Honorary Treasurer Dr Andy Brown School of Chemical and Process Engineering, University of Leeds, Leeds, LS2 9JT Tel: 0113 343 2382 Email: [email protected] Ordinary Members Dr ZiYou Li, University of Birmingham, [email protected] Dr Ana Sanchez, University of Warwick, [email protected] Dr Cornelia Rodenburg, University of Sheffield, [email protected] Dr Larry Stoter, Retired, [email protected] Mr Michael Dixon, Hitachi Europe, [email protected] Prof Jun Yuan, University of York, [email protected] Dr Donald MacLaren, University of Glasgow, [email protected] Dr Sarah Karimi, JEOL Ltd, [email protected] Co-opted Dr. -

Nanometer Resolution Elemental Mapping in Graphene-Based TEM
Nanometer Resolution Elemental Mapping in Graphene-based TEM Liquid Cells Daniel J. Kelly,†§, Mingwei Zhou‡,§, Nick Clark†,§, Matthew J. Hamer‡,§, Edward A. Lewis†, Alexander M. Rakowski†,§, Sarah J. Haigh*,†,§, and Roman V. Gorbachev*,‡,§ †School of Materials, University of Manchester, Oxford Road, Manchester, M13 9PL, UK ‡School of Physics and Astronomy, University of Manchester, Oxford Road, Manchester, M13 9PL, UK §National Graphene Institute, University of Manchester, Oxford Road, Manchester, M13 9PL, UK Keywords: EDX spectrum imaging, in situ TEM, graphene liquid cells, nanoparticles, Van der Waals heterostructures We demonstrate a new design of graphene liquid cell consisting of a thin lithographically patterned hexagonal boron nitride crystal encapsulated from both sides with graphene windows. The ultra-thin window liquid cells produced have precisely controlled volumes and thicknesses, and are robust to repeated vacuum cycling. This technology enables exciting new opportunities for liquid cell studies, providing a reliable platform for high resolution transmission electron microscope imaging and spectral mapping. The presence of water was confirmed using electron 1 energy loss spectroscopy (EELS) via the detection of the oxygen K-edge and measuring the thickness of full and empty cells. We demonstrate the imaging capabilities of these liquid cells by tracking the dynamic motion and interactions of small metal nanoparticles with diameters of 0.5-5 nm. We further present an order of magnitude improvement in the analytical capabilities compared to previous liquid cell data, with 1 nm spatial resolution elemental mapping achievable for liquid encapsulated bimetallic nanoparticles using energy dispersive X-ray spectroscopy (EDXS). One of the most attractive and unique capabilities of the scanning transmission electron microscope (STEM) is its ability to perform high spatial resolution elemental analysis through EDX and EEL spectroscopies.