Mutation—The Engine of Evolution: Studying Mutation and Its Role in the Evolution of Bacteria
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

The Transcriptional Landscape of a Rewritten Bacterial Genome Reveals Control Elements and Genome Design Principles ✉ ✉ Mariëlle J
ARTICLE https://doi.org/10.1038/s41467-021-23362-y OPEN The transcriptional landscape of a rewritten bacterial genome reveals control elements and genome design principles ✉ ✉ Mariëlle J. F. M. van Kooten 1 , Clio A. Scheidegger1, Matthias Christen1 & Beat Christen 1 Sequence rewriting enables low-cost genome synthesis and the design of biological systems with orthogonal genetic codes. The error-free, robust rewriting of nucleotide sequences can 1234567890():,; be achieved with a complete annotation of gene regulatory elements. Here, we compare transcription in Caulobacter crescentus to transcription from plasmid-borne segments of the synthesized genome of C. ethensis 2.0. This rewritten derivative contains an extensive amount of supposedly neutral mutations, including 123’562 synonymous codon changes. The tran- scriptional landscape refines 60 promoter annotations, exposes 18 termination elements and links extensive transcription throughout the synthesized genome to the unintentional intro- duction of sigma factor binding motifs. We reveal translational regulation for 20 CDS and uncover an essential translational regulatory element for the expression of ribosomal protein RplS. The annotation of gene regulatory elements allowed us to formulate design principles that improve design schemes for synthesized DNA, en route to a bright future of iteration- free programming of biological systems. 1 Institute of Molecular Systems Biology, Department of Biology, Eidgenössische Technische Hochschule Zürich, Zürich, Switzerland. ✉ email: [email protected]; [email protected] NATURE COMMUNICATIONS | (2021) 12:3053 | https://doi.org/10.1038/s41467-021-23362-y | www.nature.com/naturecommunications 1 ARTICLE NATURE COMMUNICATIONS | https://doi.org/10.1038/s41467-021-23362-y e can program biological systems with DNA that is In previous work, we synthesized and assembled the rewritten Wbased on native nucleotide sequences. -

Each of 3,323 Metabolic Innovations in the Evolution of E. Coli Arose Through the Horizontal Transfer of a Single DNA Segment
Each of 3,323 metabolic innovations in the evolution of E. coli arose through the horizontal transfer of a single DNA segment Tin Yau Panga,b and Martin J. Lerchera,b,1 aInstitute for Computer Science, Heinrich Heine University Düsseldorf, 40225 Düsseldorf, Germany; and bDepartment of Biology, Heinrich Heine University Düsseldorf, 40225 Düsseldorf, Germany Edited by W. Ford Doolittle, Dalhousie University, Halifax, Nova Scotia, Canada, and approved November 15, 2018 (received for review October 31, 2017) Even closely related prokaryotes often show an astounding to efficiently metabolize nutrient sources is an essential determi- diversity in their ability to grow in different nutritional environ- nant of bacterial fitness (12), and flux balance analysis (FBA) has ments. It has been hypothesized that complex metabolic adapta- been established as a robust and reliable modeling framework for tions—those requiring the independent acquisition of multiple the prediction of this ability (13, 14). new genes—can evolve via selectively neutral intermediates. A computational analysis of approximate metabolic models However, it is unclear whether this neutral exploration of pheno- generated automatically from genome sequences suggested that type space occurs in nature, or what fraction of metabolic adap- within-species phenotypic divergence is almost instantaneous, tations is indeed complex. Here, we reconstruct metabolic models whereas divergence between genera is gradual or “clock-like” for the ancestors of a phylogeny of 53 Escherichia coli strains, (12). Accordingly, the genetic distance calculated from multi- linking genotypes to phenotypes on a genome-wide, macroevolu- tionary scale. Based on the ancestral and extant metabolic models, locus sequence typing data is a weak indicator of how similar two we identify 3,323 phenotypic innovations in the history of the E. -

Microbial Evolution and Diversity
PART V Microbial Evolution and Diversity This material cannot be copied, disseminated, or used in any way without the express written permission of the publisher. Copyright 2007 Sinauer Associates Inc. The objectives of this chapter are to: N Provide information on how bacteria are named and what is meant by a validly named species. N Discuss the classification of Bacteria and Archaea and the recent move toward an evolutionarily based, phylogenetic classification. N Describe the ways in which the Bacteria and Archaea are identified in the laboratory. This material cannot be copied, disseminated, or used in any way without the express written permission of the publisher. Copyright 2007 Sinauer Associates Inc. 17 Taxonomy of Bacteria and Archaea It’s just astounding to see how constant, how conserved, certain sequence motifs—proteins, genes—have been over enormous expanses of time. You can see sequence patterns that have per- sisted probably for over three billion years. That’s far longer than mountain ranges last, than continents retain their shape. —Carl Woese, 1997 (in Perry and Staley, Microbiology) his part of the book discusses the variety of microorganisms that exist on Earth and what is known about their characteris- Ttics and evolution. Most of the material pertains to the Bacteria and Archaea because there is a special chapter dedicated to eukaryotic microorganisms. Therefore, this first chapter discusses how the Bacte- ria and Archaea are named and classified and is followed by several chapters (Chapters 18–22) that discuss the properties and diversity of the Bacteria and Archaea. When scientists encounter a large number of related items—such as the chemical elements, plants, or animals—they characterize, name, and organize them into groups. -

Antibiotic Resistance and the Evolution of Bacteria Is There a Human Tit Locus?
21 1 57 _N~_~___ v_o_L._m __ -~--~_L_ ~-----------------------NEWSANDVIEWS-------------------------------------'- Microbiology species exhibiting resistance, as the use of the agent continued. It became clear that two processes are in Antibiotic resistance and the volved in the evolution of resistant popula tions: the 'invention' of the resistance genes themselves, and their multiplication evolution of bacteria and spread. Relatively little is known even from Mark Richmond now about the first of the two stages, though there are some clues. As for these A PAPER by Victoria Hughes and Naomi within the population and capable of being cond, the mechanisms of genetic exchange Datta in this week's issue of Nature (p.725) transferred within the members of the discussed above, coupled with selection, fills a gap in our knowledge of the population. Furthermore, that integration can provide a complete explanation. emergence of antibiotic resistance in and excision ofplasmids into and from the One part of this story has, however, bacteria. The study makes use of a chromosome sometimes transferred blocks always been taken for granted, and it is remarkable collection of bacteria made of information from the chromosomal to here that the paper appearing in this week's between 1917 and 1954-before the use of the extrachromosomal state underlined the issue of Nature finally provides definitive antibiotics became widespread and at a fact that a bacterial population as a whole evidence. For the scenario outlined above time when little was known of bacterial had considerably genetic fluidity even if, to be correct, bacterial strains isolated genetics - and kept in sealed vessels since under normal circumstances, this fluidity before the advent of large-scale use of anti that period. -

Horizontal Gene Flow Into Geobacillus Is Constrained by the Chromosomal Organization of Growth and Sporulation
bioRxiv preprint doi: https://doi.org/10.1101/381442; this version posted August 2, 2018. The copyright holder for this preprint (which was not certified by peer review) is the author/funder, who has granted bioRxiv a license to display the preprint in perpetuity. It is made available under aCC-BY 4.0 International license. Horizontal gene flow into Geobacillus is constrained by the chromosomal organization of growth and sporulation Alexander Esin1,2, Tom Ellis3,4, Tobias Warnecke1,2* 1Molecular Systems Group, Medical Research Council London Institute of Medical Sciences, London, United Kingdom 2Institute of Clinical Sciences, Faculty of Medicine, Imperial College London, London, United Kingdom 3Imperial College Centre for Synthetic Biology, Imperial College London, London, United Kingdom 4Department of Bioengineering, Imperial College London, London, United Kingdom *corresponding author ([email protected]) 1 bioRxiv preprint doi: https://doi.org/10.1101/381442; this version posted August 2, 2018. The copyright holder for this preprint (which was not certified by peer review) is the author/funder, who has granted bioRxiv a license to display the preprint in perpetuity. It is made available under aCC-BY 4.0 International license. Abstract Horizontal gene transfer (HGT) in bacteria occurs in the context of adaptive genome architecture. As a consequence, some chromosomal neighbourhoods are likely more permissive to HGT than others. Here, we investigate the chromosomal topology of horizontal gene flow into a clade of Bacillaceae that includes Geobacillus spp. Reconstructing HGT patterns using a phylogenetic approach coupled to model-based reconciliation, we discover three large contiguous chromosomal zones of HGT enrichment. -

Section 4. Guidance Document on Horizontal Gene Transfer Between Bacteria
306 - PART 2. DOCUMENTS ON MICRO-ORGANISMS Section 4. Guidance document on horizontal gene transfer between bacteria 1. Introduction Horizontal gene transfer (HGT) 1 refers to the stable transfer of genetic material from one organism to another without reproduction. The significance of horizontal gene transfer was first recognised when evidence was found for ‘infectious heredity’ of multiple antibiotic resistance to pathogens (Watanabe, 1963). The assumed importance of HGT has changed several times (Doolittle et al., 2003) but there is general agreement now that HGT is a major, if not the dominant, force in bacterial evolution. Massive gene exchanges in completely sequenced genomes were discovered by deviant composition, anomalous phylogenetic distribution, great similarity of genes from distantly related species, and incongruent phylogenetic trees (Ochman et al., 2000; Koonin et al., 2001; Jain et al., 2002; Doolittle et al., 2003; Kurland et al., 2003; Philippe and Douady, 2003). There is also much evidence now for HGT by mobile genetic elements (MGEs) being an ongoing process that plays a primary role in the ecological adaptation of prokaryotes. Well documented is the example of the dissemination of antibiotic resistance genes by HGT that allowed bacterial populations to rapidly adapt to a strong selective pressure by agronomically and medically used antibiotics (Tschäpe, 1994; Witte, 1998; Mazel and Davies, 1999). MGEs shape bacterial genomes, promote intra-species variability and distribute genes between distantly related bacterial genera. Horizontal gene transfer (HGT) between bacteria is driven by three major processes: transformation (the uptake of free DNA), transduction (gene transfer mediated by bacteriophages) and conjugation (gene transfer by means of plasmids or conjugative and integrated elements). -
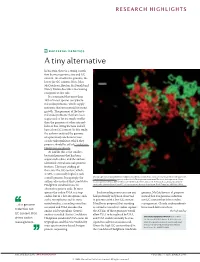
Bacterial Genetics a Tiny Alternative
RESEARCH HIGHLIGHTS BACTERIAL GENETICS A tiny alternative In bacteria, there is a strong correla- tion between genome size and GC content: the smaller the genome, the lower the GC content. Now, John McCutcheon, Bradon McDonald and Nancy Moran describe a fascinating exception to this rule. It is estimated that more than 10% of insect species carry bacte- rial endosymbionts, which supply nutrients that are essential for insect growth. The genomes of the bacte- rial endosymbionts that have been sequenced so far are much smaller than the genomes of other intracel- lular or free-living bacteria and all have a low GC content. In this study, the authors analysed the genome of a previously uncharacterized cicada endosymbiont, which they propose should be called Candidatus Hodgkinia cicadicola. At 144 kb, this is the smallest bacterial genome that has been sequenced to date, and the authors identified several unusual genomic features. The most striking of these was the GC content, which, at 58%, is unusually high for such Micrograph showing Candidatus Hodgkinia cicadicola (red) in close association with another endosymbiont, a small genome. Intriguingly, the Candidatus Sulcia muelleri (green) in the cicada Diceroprocta semicincta. The scale bar represents 10 µm. authors also noticed that Candidatus Image reproduced from McCutcheon, J. P., McDonald, B. R. & Moran, N. P. Origin of an alternative genetic Hodgkinia cicadicola uses an code in the extremely small and GC-rich genome of a bacterial symbiont. PLoS Genet. 5, e1000565 (2009). alternative genetic code. In most species the codon UGA is a stop Such recoding events are rare and genome, McCutcheon et al. -

Synthetic Biology Projects in Vitro
Downloaded from genome.cshlp.org on September 25, 2021 - Published by Cold Spring Harbor Laboratory Press Review Synthetic biology projects in vitro Anthony C. Forster1,3 and George M. Church2,3 1Department of Pharmacology and Vanderbilt Institute of Chemical Biology, Vanderbilt University Medical Center, Nashville, Tennessee 37232, USA; 2Department of Genetics, Harvard Medical School, Boston, Massachusetts 02115, USA Advances in the in vitro synthesis and evolution of DNA, RNA, and polypeptides are accelerating the construction of biopolymers, pathways, and organisms with novel functions. Known functions are being integrated and debugged with the aim of synthesizing life-like systems. The goals are knowledge, tools, smart materials, and therapies. Synthetic biology projects (SBPs) an action plan to regulate world suppliers of DNA synthesizers, DNA precursors, and oligos (Church 2004). Ethical and safety The basic elements of chemistry and biology are few, but the issues have been, and must continue to be, regulated (Cho et al. synthetic combinations are unlimited and awe inspiring. The 1999). first international conference on synthetic biology charted its Several groups have proposed to create bacteria with chro- goals as understanding and utilizing life’s diverse solutions to mosomes synthesized entirely from synthetic oligos. This might process information, materials, and energy (Silver and Way 2004) be done stepwise (Posfai et al. 2006) or by inactivating the en- (http://syntheticbiology.org). As a bonus, genetic systems are dogenous bacterial chromosome and then somehow transform- biocompatible, renewable, and can be optimized by Darwinian ing and rebooting the bacterium with an entire in vitro- selections. SBPs entail the complex manipulation of replicating synthesized genome. -

Assessment of the Bimodality in the Distribution of Bacterial Genome Sizes
The ISME Journal (2017) 11, 821–824 © 2017 International Society for Microbial Ecology All rights reserved 1751-7362/17 www.nature.com/ismej SHORT COMMUNICATION Assessment of the bimodality in the distribution of bacterial genome sizes Hyun S Gweon, Mark J Bailey and Daniel S Read Centre for Ecology & Hydrology, Wallingford, UK Bacterial genome sizes have previously been shown to exhibit a bimodal distribution. This phenomenon has prompted discussion regarding the evolutionary forces driving genome size in bacteria and its ecological significance. We investigated the level of inherent redundancy in the public database and the effect it has on the shape of the apparent bimodal distribution. Our study reveals that there is a significant bias in the genome sequencing efforts towards a certain group of species, and that correcting the bias using species nomenclature and clustering of the 16S rRNA gene, results in a unimodal rather than the previously published bimodal distribution. The true genome size distribution and its wider ecological implications will soon emerge as we are currently witnessing rapid growth in the number of sequenced genomes from diverse environmental niches across a range of habitats at an unprecedented rate. The ISME Journal (2017) 11, 821–824; doi:10.1038/ismej.2016.142; published online 11 November 2016 Significant progress has been made in understanding Wolf in 2008, where it was reported that bacterial interactions between ecology and genome evolution genome sizes show a bimodal distribution (Koonin in prokaryotes. -

Mechanisms Of, and Barriers To, Horizontal Gene Transfer Between Bacteria
FOCUS ON HORIZONTAL GENE TRANSFER MECHANISMS OF, AND BARRIERS TO, HORIZONTAL GENE TRANSFER BETWEEN BACTERIA Christopher M. Thomas* and Kaare M. Nielsen‡ Abstract | Bacteria evolve rapidly not only by mutation and rapid multiplication, but also by transfer of DNA, which can result in strains with beneficial mutations from more than one parent. Transformation involves the release of naked DNA followed by uptake and recombination. Homologous recombination and DNA-repair processes normally limit this to DNA from similar bacteria. However, if a gene moves onto a broad-host-range plasmid it might be able to spread without the need for recombination. There are barriers to both these processes but they reduce, rather than prevent, gene acquisition. The first evidence that horizontal gene transfer (HGT) informational genes of the central cellular machinery could occur was the recognition that virulence deter- such as DNA replication, transcription or translation minants could be transferred between pneumococci in tend not to spread rapidly, even if they confer anti biotic infected mice, a phenomenon that was later shown to resistance, compared for example to single-function- be mediated by the uptake of the genetic material DNA resistance determinants such as β-lactamases or in a process called transformation1. The subsequent aminoglycoside-modifying enzymes. However, the identification of gene transfer mediated by both plas- nature of the transfer mechanism can also determine the mids and viruses and the recognition of transposable organisms and genes that are most often involved. The elements provided the stepping stones to our current purpose of this review is to describe some of the mecha- picture of gene flux and the importance of mobile nisms that lead to horizontal gene acquisitions with a genetic elements2. -

Selection and Robustness in Bacterial Genome Evolution Seila Omer University of Connecticut, [email protected]
University of Connecticut OpenCommons@UConn Doctoral Dissertations University of Connecticut Graduate School 12-16-2016 Selection and Robustness in Bacterial Genome Evolution Seila Omer University Of Connecticut, [email protected] Follow this and additional works at: https://opencommons.uconn.edu/dissertations Recommended Citation Omer, Seila, "Selection and Robustness in Bacterial Genome Evolution" (2016). Doctoral Dissertations. 1317. https://opencommons.uconn.edu/dissertations/1317 Selection and Robustness in Bacterial Genome Evolution Seila Omer, Ph.D. University of Connecticut, 2016 The research presented in this thesis attempts to address research questions related to the role of natural selection in the evolution of bacterial genes not expressed for function and in building mutational tolerance to translational errors. Studies on evolution of protein coding DNA sequences have provided the evidence for a current paradigm in evolutionary biology: only functional genes are undergoing selection against the deleterious effects of allele variants (purifying selection). I provide evidence that similar footprints of selection can be detected in genes that are not normally expressed for function during the bacterial life cycle. Using simulations for DNA sequence evolution, I demonstrate statistically significant deviations from neutral evolution for the studied genes. I suggest that purifying selection affects both functional and non-functional genes. I propose this might be caused by the dominant toxic effects of low level translation of mutated products in bacteria, due to misfolding and misinteraction. Natural selection also acts to remove the effects of translational errors. Stop codon readthrough events are more likely to have major structural and functional effects than simple nucleotide changes. Recent research has shown that strength of selection experienced by protein-coding genes is positively correlated with the level of gene expression. -

Experimental Microbial Evolution of Extremophiles Paul H
View metadata, citation and similar papers at core.ac.uk brought to you by CORE provided by DigitalCommons@University of Nebraska University of Nebraska - Lincoln DigitalCommons@University of Nebraska - Lincoln Faculty Publications in the Biological Sciences Papers in the Biological Sciences 2016 Experimental Microbial Evolution of Extremophiles Paul H. Blum University of Nebraska-Lincoln, [email protected] Deepak Rudrappa University of Nebraska–Lincoln, [email protected] Raghuveer Singh University of Nebraska - Lincoln, [email protected] Samuel McCarthy University of Nebraska-Lincoln, [email protected] Benjamin J. Pavlik University of Nebraska- Lincoln, [email protected] Follow this and additional works at: http://digitalcommons.unl.edu/bioscifacpub Part of the Biology Commons Blum, Paul H.; Rudrappa, Deepak; Singh, Raghuveer; McCarthy, Samuel; and Pavlik, Benjamin J., "Experimental Microbial Evolution of Extremophiles" (2016). Faculty Publications in the Biological Sciences. 623. http://digitalcommons.unl.edu/bioscifacpub/623 This Article is brought to you for free and open access by the Papers in the Biological Sciences at DigitalCommons@University of Nebraska - Lincoln. It has been accepted for inclusion in Faculty Publications in the Biological Sciences by an authorized administrator of DigitalCommons@University of Nebraska - Lincoln. Published (as Chapter 22) in P. H. Rampelotto (ed.), Biotechnology of Extremophiles, Grand Challenges in Biology and Biotechnology 1, pp. 619–636. DOI 10.1007/978-3-319-13521-2_22 Copyright © 2016 Springer International Publishing Switzerland. digitalcommons.unl.edu Used by permission. Experimental Microbial Evolution of Extremophiles Paul Blum,1 Deepak Rudrappa,1 Raghuveer Singh,1 Samuel McCarthy,1 and Benjamin Pavlik2 1 School of Biological Science, University of Nebraska–Lincoln, Lincoln, NE, USA 2 Department of Chemical and Biomolecular Engineering, University of Nebraska–Lincoln, Lincoln, NE, USA Corresponding author — P.