Studies on the Permeability of Lymphatic Capillaries Lee V. Leak
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Flow Across Microvessel Walls Through the Endothelial Surface Glycocalyx and the Interendothelial Cleft
View metadata, citation and similar papers at core.ac.uk brought to you by CORE provided by Aston Publications Explorer J. Fluid Mech. (2008), vol. 601, pp. 229–252. c 2008 Cambridge University Press 229 doi:10.1017/S0022112008000530 Printed in the United Kingdom Flow across microvessel walls through the endothelial surface glycocalyx and the interendothelial cleft M. SUGIHARA-SEKI, T. AKINAGA AND T. ITANO Department of Physics, Kansai University, 3-3-35 Yamate-cho, Suita, Osaka 564-8680, Japan (Received 8 March 2007 and in revised form 9 January 2008) A mathematical model is presented for steady fluid flow across microvessel walls through a serial pathway consisting of the endothelial surface glycocalyx and the intercellular cleft between adjacent endothelial cells, with junction strands and their discontinuous gaps. The three-dimensional flow through the pathway from the vessel lumen to the tissue space has been computed numerically based on a Brinkman equation with appropriate values of the Darcy permeability. The predicted values of the hydraulic conductivity Lp, defined as the ratio of the flow rate per unit surface area of the vessel wall to the pressure drop across it, are close to experimental measurements for rat mesentery microvessels. If the values of the Darcy permeability for the surface glycocalyx are determined based on the regular arrangements of fibres with 6 nm radius and 8 nm spacing proposed recently from the detailed structural measurements, then the present study suggests that the surface glycocalyx could be much less resistant to flow compared to previous estimates by the one-dimensional flow analyses, and the intercellular cleft could be a major determinant of the hydraulic conductivity of the microvessel wall. -

The Ultrastructural Basis of Alveolar-Capillary Membrane Permeability to Peroxidase Used As a Tracer
Published Online: 23 February, 2004 | Supp Info: http://doi.org/10.1083/jcb.37.3.781 Downloaded from jcb.rupress.org on May 11, 2019 THE ULTRASTRUCTURAL BASIS OF ALVEOLAR-CAPILLARY MEMBRANE PERMEABILITY TO PEROXIDASE USED AS A TRACER EVELINE E. SCHNEEBERGER-KEELEY and MORRIS J. KARNOVSKY From the Department of Pathology, Harvard Medical School, Boston, Massachusetts 02115 ABSTRACT The permeability of the alveolar-capillary membrane to a small molecular weight protein, horseradish peroxidase (HRP), was investigated by means of ultrastructural cytochemistry. Mice were injected intravenously with HRP and sacrificed at varying intervals. Experi- ments with intranasally instilled HRP were also carried out. The tissue was fixed in formal- dehyde-glutaraldehyde fixative. Frozen sections were cut, incubated in Graham and Karnovsky's medium for demonstrating HRP activity, postfixed in OSO4, and processed for electron microscopy. 90 sec after injection, HRP had passed through endothelial junctions into underlying basement membranes, but was stopped from entering the alveolar space by zonulae occludentes between epithelial cells. HRP was demonstrated in pinocytotic vesicles of both endothelial and epithelial cells, but the role of these vesicles in net protein transport appeared to be minimal. Intranasally instilled HRP was similarly prevented from permeat- ing the underlying basement membrane by epithelial zonulae occludentes. Pulmonary endothelial intercellular clefts stained with uranyl acetate appeared to contain maculae oc- cludentes rather than zonulae occludentes. HRP did not alter the ultrastructure of these junctions. INTRODUCTION The permeability of the alveolar-capillary mem- nature of the alveolar epithelium. Later studies of brane to solutes and water has been the subject the mouse lung by Karrer (19) further clarified of many physiological studies (4, 5, 8, 11, 27, 29). -

Junctions Between Intimately Apposed Cell Membranes in the Vertebrate Brain
JUNCTIONS BETWEEN INTIMATELY APPOSED CELL MEMBRANES IN THE VERTEBRATE BRAIN M. W. BRIGHTMAN and T. S. REESE From the Sections on Neurocytology and Functional Neuroanatomy, Laboratory of Neuropathology and Neuroanatomical Sciences, National Institutes of Health, Bethesda, Maryland 20014 Downloaded from http://rupress.org/jcb/article-pdf/40/3/648/1384884/648.pdf by guest on 02 October 2021 ABSTRACT Certain junctions between ependymal cells, between astrocytes, and between some elec- trically coupled neurons have heretofore been regarded as tight, pentalaminar occlusions of the intercellular cleft. These junctions are now redefined in terms of their configuration after treatment of brain tissue in uranyl acetate before dehydration. Instead of a median dense lamina, they are bisected by a median gap 20-30 A wide which is continuous with the rest of the interspace. The patency of these "gap junctions" is further demonstrated by the penetration of horseradish peroxidase or lanthanum into the median gap, the latter tracer delineating there a polygonal substructure. However, either tracer can circumvent gap junctions because they are plaque-shaped rather than complete, circumferential belts. Tight junctions, which retain a pentalaminar appearance after uranyl acetate block treat- ment, are restricted primarily to the endothelium of parenchymal capillaries and the epi- thelium of the choroid plexus. They form rows of extensive, overlapping occlusions of the interspace and are neither circumvented nor penetrated by peroxidase and lanthanum. These junctions are morphologically distinguishable from the "labile" pentalaminar apposi- tions which appear or disappear according to the preparative method and which do not interfere with the intercellular movement of tracers. Therefore, the interspaces of the brain are generally patent, allowing intercellular movement of colloidal materials. -

Glycocalyx and Its Involvement in Clinical Pathophysiologies Akira Ushiyama1, Hanae Kataoka2 and Takehiko Iijima2*
Ushiyama et al. Journal of Intensive Care (2016) 4:59 DOI 10.1186/s40560-016-0182-z REVIEW Open Access Glycocalyx and its involvement in clinical pathophysiologies Akira Ushiyama1, Hanae Kataoka2 and Takehiko Iijima2* Abstract Vascular hyperpermeability is a frequent intractable feature involved in a wide range of diseases in the intensive care unit. The glycocalyx (GCX) seemingly plays a key role to control vascular permeability. The GCX has attracted the attention of clinicians working on vascular permeability involving angiopathies, and several clinical approaches to examine the involvement of the GCX have been attempted. The GCX is a major constituent of the endothelial surface layer (ESL), which covers most of the surface of the endothelial cells and reduces the access of cellular and macromolecular components of the blood to the surface of the endothelium. It has become evident that this structure is not just a barrier for vascular permeability but contributes to various functions including signal sensing and transmission to the endothelium. Because GCX is a highly fragile and unstable layer, the image had been only obtained by conventional transmission electron microscopy. Recently, advanced microscopy techniques have enabled direct visualization of the GCX in vivo, most of which use fluorescent-labeled lectins that bind to specific disaccharide moieties of glycosaminoglycan (GAG) chains. Fluorescent-labeled solutes also enabled to demonstrate vascular leakage under the in vivo microscope. Thus, functional analysis of GCX is advancing. A biomarker of GCX degradation has been clinically applied as a marker of vascular damage caused by surgery. Fragments of the GCX, such as syndecan-1 and/or hyaluronan (HA), have been examined, and their validity is now being examined. -

Cardiovascular System
Cardiovascular System BLOOD VESSELS Blood Vessels Delivery system of dynamic structures Closed system Arteries Carry blood away from the heart Capillaries Contact tissue cells and directly serve cellular needs Veins Carry blood toward the heart Venous system Arterial system Large veins Heart (capacitance vessels) Elastic arteries Large (conducting lymphatic vessels) vessels Lymph node Muscular arteries (distributing Lymphatic vessels) system Small veins (capacitance Arteriovenous vessels) anastomosis Lymphatic Sinusoid capillary Arterioles (resistance vessels) Postcapillary Terminal arteriole venule Metarteriole Thoroughfare Capillaries Precapillary sphincter channel (exchange vessels) Figure 19.2 Common Capillary beds of carotid arteries head and to head and upper limbs subclavian arteries to upper limbs Superior vena cava Aortic arch Aorta RA LA RV LV Azygos Thoracic system aorta Venous Arterial drainage blood Inferior vena Capillary beds of cava mediastinal structures and thorax walls Diaphragm Abdominal aorta Capillary beds of Inferior digestive viscera, vena spleen, pancreas, cava kidneys Capillary beds of gonads, pelvis, and lower limbs Figure 19.20 Tissue Layers Tunica interna Tunica media Tunica externa Tunica intima (interna) • Endothelium Valve • Subendothelial layer Internal elastic lamina Tunica media (smooth muscle and elastic fibers) External elastic lamina Tunica externa (collagen fibers) Lumen Lumen Capillary Vein Artery network Basement membrane Endothelial cells (b) Capillary Figure 19.1b Vein Artery (a) Figure -

Electron Microscopic Study of Lymphatic Capillaries in the Removal of Connective Tissue Fluids and Particulate Substances*
Electron Microscopic Study of Lymphatic Capillaries in the Removal of Connective Tissue Fluids and Particulate Substances* L. V. Leak and J. F. Burke Departments of Anatomy and Surgery, Harvard Medical School and the General Surgical Services, Massachusetts General Hospital, Boston, Massachusetts 0 2114 USA Lymphology 1, 39·52 (1968) The removal of connective tissue fluids by the lymphatic capillaries is of fundamen tal importance in the normal maintenance of fluid balance for body tissues. In addition to restoring extravascular proteins back to the blood stream, the lymphatic capillaries are important in the response of the organism to infection and in the spread of disease to various parts of the body. The early studies of Hudack and McMaster (16) and Drinker and Field (10) clearly demonstrated that an intradermal injection of vital dyes or colloidal opaque particles readily passed from connective tissue spaces into lym phatic capillaries. More recent studies (6, 19, 20) indicate that fluids and particulate substances gain entrance into lumina of lymphatics via one of two pathways, i.e. (1) across the intercellular cleft of patent junction and (2) across the lymphatic endo thelium within pinocytotic vesicles. In order to gain additional information on the mechanisms involved in this transfer of fluids and particulate substances into lymphatics, the present study was designed to investigate this problem by studying the fate of particulate substances after their injection into the connective tissue. Since intradermally injected colloidal particles are not engulfed by endothelia of blood capillaries, but are rapidly ingested by those of the lymphatics, the latter are identified by the presence of opaque particles within the endothelium as well as their lumina. -

A9e7f208140ee926c3a928be02f
GAP JUNCTIONS IN ARTERIAL ENDOTHELIUM ISTVAN HµTTNER, MICHEL BOUTET, and ROBERT H. MORE . From the Department of Pathology, McGill University, Montreal, Quebec, Canada INTRODUCTION external iliac artery, in small arteries in the vicinity of the aorta and carotid artery, as well as in intra- Tight junctions are well characterized membrane mural coronary arteries . The tissues were obtained specializations in vascular endothelium . These from 12 male Sprague-Dawley rats weighing 300- structures seal the interspace between adjacent 400 g each. endothelial cells, either completely, as in cerebral vessels (10), or incompletely, as in striated and Preparation for Electron Microscopy cardiac muscle capillaries (8, 19) as well as in non- cerebral arteries (4, 5) . Gap junctions in vascular Fixation was initiated by perfusion of Karnovsky's fixative (7) containing 1 % freshly prepared parafor- endothelium have not been described in the litera- maldehyde and 1 .25% purified glutaraldehyde in ture as far as we know. Extensive membrane ap- 0.1 N sodium cacodylate buffer (pH 7.4), with 5% positions were recently reported in the endo- sucrose (final osmolality : -750 mosmol) . Perfusion thelium of rat thoracic aorta, but these structures was started under ether anesthesia through the left were interpreted as tight junctions (16) . The ventricle of the heart, and maintained for 10 min at present study, utilizing uranyl-treated and lan- 120 mm Hg pressure while allowing the fixative to thanum-permeated tissue preparations, identified escape through the vena cava inferior . Segments gap junctions in the endothelium of selected from the large arteries cited above, as well as pieces arterial regions . The possible functional sig- from the right ventricular myocardium were then nificance of the finding is discussed . -

Molecular Anatomy of Interendothelial Junctions in Human Blood-Brain Barrier Microvessels
FOLIA HISTOCHEMICA ET CYTOBIOLOGICA Vol. 42, No. 2, 2004 pp. 67-75 Molecular anatomy of interendothelial junctions in human blood-brain barrier microvessels Andrzej W. Vorbrodt and Danuta H. Dobrogowska New York State Office of Mental Retardation and Developmental Disabilities, Institute for Basic Research in Developmental Disabilities, Staten Island, New York, USA Abstract: Immunogold cytochemical procedure was used to study the localization at the ultrastructural level of interendothe- lial junction-associated protein molecules in the human brain blood microvessels, representing the anatomic site of the blood-brain barrier (BBB). Ultrathin sections of Lowicryl K4M-embedded biopsy specimens of human cerebral cortex obtained during surgical procedures were exposed to specific antibodies, followed by colloidal gold-labeled secondary antibodies. All tight junction-specific integral membrane (transmembrane) proteins - occludin, junctional adhesion molecule (JAM-1), and claudin-5 - as well as peripheral zonula occludens protein (ZO-1) were highly expressed. Immunoreactivity of the adherens junction-specific transmembrane protein VE-cadherin was of almost similar intensity. Immunolabeling of the adherens junction-associated peripheral proteins - α-catenin, β-catenin, and p120 catenin - although positive, was evidently less intense. The expression of γ-catenin (plakoglobin) was considered questionable because solitary immunosignals (gold particles) appeared in only a few microvascular profiles. Double labeling of some sections made possible to observe strict colocalization of the junctional molecules, such as occludin and ZO-1 or JAM-1 and VE-cadherin, in the interendothelial junctions. We found that in human brain microvessels, the interendothelial junctional complexes contain molecular components specific for both tight and adherens junctions. It is assumed that the data obtained can help us find the immunodetectable junctional molecules that can serve as sensitive markers of normal or abnormal function of the BBB. -

Microvascular Fluid Exchange and the Revised Starling Principle
Cardiovascular Research (2010) 87, 198–210 SPOTLIGHT REVIEW doi:10.1093/cvr/cvq062 Microvascular fluid exchange and the revised Starling principle J. Rodney Levick 1 and C. Charles Michel 2* 1Physiology, Basic Medical Sciences, St George’s Hospital Medical School, London SW17 0RE, UK; and 2Department of Bioengineering, Imperial College, Exhibition Road, London SW7 2AZ, UK Received 30 November 2009; revised 4 February 2010; accepted 18 February 2010; online publish-ahead-of-print 3 March 2010 Microvascular fluid exchange (flow Jv) underlies plasma/interstitial fluid (ISF) balance and oedematous swelling. The traditional form of Starling’s principle has to be modified in light of insights into the role of ISF pressures and the recognition of the glycocalyx as the semi- permeable layer of endothelium. Sum-of-forces evidence and direct observations show that microvascular absorption is transient in most tissues; slight filtration prevails in the steady state, even in venules. This is due in part to the inverse relation between filtration rate and ISF plasma protein concentration; ISF colloid osmotic pressure (COP) rises as Jv falls. In some specialized regions (e.g. kidney, intestinal mucosa), fluid absorption is sustained by local epithelial secretions, which flush interstitial plasma proteins into the lymphatic system. The low rate of filtration and lymph formation in most tissues can be explained by standing plasma protein gradients within the intercellular cleft of continuous capillaries (glycocalyx model) and around fenestrations. Narrow breaks in the junctional strands of the cleft create high local outward fluid velocities, which cause a disequilibrium between the subglycocalyx space COP and ISF COP. -

The Ultrastructural Basis of Transcapillary Exchanges
The Ultrastructural Basis of Transcapillary Exchanges MORRIS J. KARNOVSKY From the Department of Pathology, Harvard Medical School, Boston, Massachusetts 02115 ABSTRACT A brief survey is given of current views correlating the ultra- structural and permeability characteristics of capillaries. Observations based on the use of peroxidase (mol wt 40,000), as an in vivo, and colloidal lanthanum, as an in vitro, ultrastructural tracer, are presented. In capillaries with "con- tinuous" endothelium, the endothelial intercellular junctions are thought to be permeable to the tracers, and are regarded as maculae occludentes rather than zonulae occludentes, with a gap of about 40 A in width between the maculae. Some evidence for vesicular transport is also presented. It is inferred that the cell junctions are the morphological equivalent of the small-pore system, and the vesicles the equivalent of the large-pore system. Peroxidase does not appar- ently cross brain capillaries: the endothelial cell junctions are regarded as zonulae occludentes, and vesicles do not appear to transport across the endo- thelium. This is regarded as the morphological equivalent of the blood-brain barrier for relatively large molecules. The tracers appear to permeate the fen- estrae of fenestrated capillaries, and the high permeability of these capillaries to large molecules is attributed to the fenestrae. Capillaries with discontinuous endothelium readily allow passage of the tracers through the intercellular gaps. A continuous basement membrane may act as a relatively coarse filter for large molecules. In general, the morphology of capillaries correlates well with physiological observations. Since the introduction of the electron microscope and the elucidation of the ultrastructure of cells, the physiologist, biochemist, and morphologist have been able to make considerable advances in the correlation of structure and function, at least at the macromolecular level. -

A Cellular Scale Study of Low Density Lipoprotein Concentration Polarisation in Arteries
A Cellular Scale Study of Low Density Lipoprotein Concentration Polarisation in Arteries by P. E. Vincent Department of Aeronautics Imperial College London Prince Consort Road London SW7 2BY This thesis is submitted for the degree of Doctor of Philosophy of the University of London 2009 Declaration This is to certify that the work presented in this thesis has been carried out at Imperial College London and has not been previously submitted to any other university or technical institution for a degree or award. I further certify that all material in this thesis which is not my own work has been properly acknowledged. P. E. Vincent Abstract Uptake of Low Density Lipoprotein (LDL) by the arterial wall is likely to play a key role in the process of atherogenesis, which occurs non-uniformly within the ar- terial vasculature. A particular process that may cause vascular scale heterogeneity in the rate of transendothelial LDL transport is the formation of a flow-dependent LDL concentration polarisation layer adjacent to the luminal surface of the arte- rial endothelium. In this thesis the effects of cellular scale endothelial features on such LDL concentration polarisation are investigated using an idealised theoretical model. Specifically, the effect of a spatially heterogeneous transmural water flux is considered (flowing only through intercellular clefts), as well as the effect of the endothelial glycocalyx layer (EGL). The idealised model is implemented using both analytical techniques and the spectral/hp element method. A range of scenarios are considered, including those were no EGL is present, those where an EGL is present but LDL cannot penetrate into it, and finally those where an EGL is present and LDL can penetrate into it. -
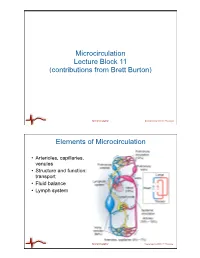
Elements of Microcirculation
Microcirculation Lecture Block 11 (contributions from Brett Burton) Microcirculation Bioengineering 6000 CV Physiology Elements of Microcirculation • Arterioles, capillaries, venules • Structure and function: transport • Fluid balance • Lymph system Microcirculation Bioengineering 6000 CV Physiology Vessels of the Circulatory System Aorta Artery Vein Vena Cava Arteriole Capillary Venule Diameter 25 mm 4 mm 5 mm 30 mm 30 µm 8 µm 20 µm Wall 2 mm 1 mm 0.5 mm 1.5 mm 6 µm 0.5 µm 1 µm thickness Endothelium Elastic tissue Smooth Muscle Fibrous Tissue Microcirculation Bioengineering 6000 CV Physiology Built to Transport Arteries and Veins Arterioles, Venules, Capillaries Microcirculation Bioengineering 6000 CV Physiology Structure of the Capillary System • Arterioles – < 40 µm diameter – thick smooth muscle layer precapillary • Metarterioles sphincter – connect arterioles and capillaries – discontinuous smooth muscle layer – serve as shunts • Capillaries – approx. 10 billion in the body – 500-700 m2 surface area – <30 µm from any cell to a cap – 4-9 µm diameter, 1 mm long – no smooth muscle, contractile endothelial cells but not clear if functional • Venules – larger than arterioles – weak smooth muscle layer Microcirculation Bioengineering 6000 CV Physiology Capillary Wall Structure • Intercellular cleft (Junctions): – 6-7 nm space between adjacent endothelial cells Nucleus – site of transport, although only 1/1000th of surface area – reduced in size in brain (blood-brain barrier) • Discontinuous endothelium – large spaces between endothelial