ELEMENT STEWARDSHIP ABSTRACT for Ligustrum Spp
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

'Ivory Silk' Japanese Tree Lilac
Fact Sheet ST-611 October 1994 Syringa reticulata ‘Ivory Silk’ ‘Ivory Silk’ Japanese Tree Lilac1 Edward F. Gilman and Dennis G. Watson2 INTRODUCTION Although a Lilac, this member of the species is quite different in appearance than those with which gardeners are more familiar (Fig. 1). Its upright habit varies from symmetrical to irregular but is more consistent than the species. Cultivars including ‘Ivory Silk’ and ‘Summer Snow’ could be used instead of the species due to the more consistent habit and more flowers. ‘Ivory Silk’ grows well only in USDA hardiness zones 3 through six (perhaps into 7) and has an oval or pyramidal form when young but spreads to a rounded shape as it grows older. This is a very large shrub or small tree, reaching a height of about 20 to 30 feet with a 15-foot-spread. The huge clusters of creamy white flowers, borne in early summer for about two weeks, are the main ornamental feature but lack the fragrance of the spring-blooming Lilacs -- this Lilac’s fragrance is more suggestive of Privet. GENERAL INFORMATION Scientific name: Syringa reticulata ‘Ivory Silk’ Pronunciation: sih-RING-guh reh-tick-yoo-LAY-tuh Common name(s): ‘Ivory Silk’ Japanese Tree Lilac Family: Oleaceae Figure 1. Mature ‘Ivory Silk’ Japanese Tree Lilac. USDA hardiness zones: 3A through 7A (Fig. 2) Origin: not native to North America sidewalk cutout (tree pit); residential street tree; tree Uses: container or above-ground planter; large has been successfully grown in urban areas where air parking lot islands (> 200 square feet in size); wide pollution, poor drainage, compacted soil, and/or tree lawns (>6 feet wide); medium-sized tree lawns drought are common (4-6 feet wide); recommended for buffer strips around Availability: somewhat available, may have to go out parking lots or for median strip plantings in the of the region to find the tree highway; near a deck or patio; screen; trainable as a standard; narrow tree lawns (3-4 feet wide); specimen; 1. -

(Insecta: Coleoptera: Scarabaeidae : Cetoniinae : Tribe, Trichiini)1 Brandon Jones and Andrea Lucky2
EENY-704 Delta Flower Beetle Trigonopeltastes delta (Forster 1771) (Insecta: Coleoptera: Scarabaeidae : Cetoniinae : Tribe, Trichiini)1 Brandon Jones and Andrea Lucky2 Introduction The delta flower beetle, Trigonopeltastes delta (Forster), is a member of the scarab beetle family Scarabaeidae, in the subfamily Cetoniinae. This subfamily is commonly known as flower or fruit chafers because their diet consists mostly of decomposing fruits or pollen (Cave and Ratcliffe 2008). Trigonopeltastes delta belongs to the tribe Trichiini, which contains mostly flower-frequenting species. Although this species is commonly encountered where it occurs, many details of its life cycle and its potential economic importance remain poorly studied. Like many other cetoniines, the delta flower beetle has bright colors and distinctive patterns that distinguish it from other similar species (Figure 1). The delta flower beetle is one of two species in Florida, but while Trigonopeltastes delta is a familiar sight, Trigono- Figure 1. Adult Trigonopeltastes delta (Forster) (dorsal view). peltastes floridana is extremely rare (Woodruff 1960). While Credits: Mike Quinn, TexasEnto.net they are superficially similar, these species are distinguished by distinctive yellow markings on the pronotum. Trigono- Etymology and Synonymy peltastes delta bears a triangular mark whereas Trigono- The name Trigonopeltastes delta is Greek in origin and peltastes floridana has a U- or V-shaped mark. describes the pronotal markings of the species. Trigon translates to triangle, pelt translates to a shield, and delta originates from the letter Δ, or delta. The Greek symbol for delta is a triangle, which resembles the beetle’s pronotal marking, and accounts for its common name. 1. This document is EENY-704, one of a series of the Department of Entomology and Nematology, UF/IFAS Extension. -

Approved Plant List 10/04/12
FLORIDA The best time to plant a tree is 20 years ago, the second best time to plant a tree is today. City of Sunrise Approved Plant List 10/04/12 Appendix A 10/4/12 APPROVED PLANT LIST FOR SINGLE FAMILY HOMES SG xx Slow Growing “xx” = minimum height in Small Mature tree height of less than 20 feet at time of planting feet OH Trees adjacent to overhead power lines Medium Mature tree height of between 21 – 40 feet U Trees within Utility Easements Large Mature tree height greater than 41 N Not acceptable for use as a replacement feet * Native Florida Species Varies Mature tree height depends on variety Mature size information based on Betrock’s Florida Landscape Plants Published 2001 GROUP “A” TREES Common Name Botanical Name Uses Mature Tree Size Avocado Persea Americana L Bahama Strongbark Bourreria orata * U, SG 6 S Bald Cypress Taxodium distichum * L Black Olive Shady Bucida buceras ‘Shady Lady’ L Lady Black Olive Bucida buceras L Brazil Beautyleaf Calophyllum brasiliense L Blolly Guapira discolor* M Bridalveil Tree Caesalpinia granadillo M Bulnesia Bulnesia arboria M Cinnecord Acacia choriophylla * U, SG 6 S Group ‘A’ Plant List for Single Family Homes Common Name Botanical Name Uses Mature Tree Size Citrus: Lemon, Citrus spp. OH S (except orange, Lime ect. Grapefruit) Citrus: Grapefruit Citrus paradisi M Trees Copperpod Peltophorum pterocarpum L Fiddlewood Citharexylum fruticosum * U, SG 8 S Floss Silk Tree Chorisia speciosa L Golden – Shower Cassia fistula L Green Buttonwood Conocarpus erectus * L Gumbo Limbo Bursera simaruba * L -
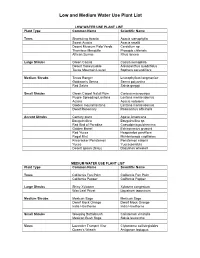
Low and Medium Water Use Plant List
Low and Medium Water Use Plant List LOW WATER USE PLANT LIST Plant Type Common Name Scientific Name Trees Shoestring Acacia Acacia stenophylla Sweet Acacia Acacia smallii Desert Museum Palo Verde Cercidium sp. Thornless Mesquite Prosopis chilensis African Sumac Rhus lancea Large Shrubs Green Cassia Cassia nemophila Desert Honeysuckle Anisacanthus quadrifidus Texas Mountain Laurel Sophora secundiflora Medium Shrubs Texas Ranger Leucophyllum langmaniae Goldman’s Senna Senna polyantha Red Salvia Salvia greggii Small Shrubs Green Carpet Natal Plum Carissa macrocarpa Purple Spreading Lantana Lantana montevidensis Acacia Acacia redolens Golden mound lantana Lantana montevidensis Dwarf Rosemary Rosmarinus officinalis Accent Shrubs Century plant Agave Americana Bougainvillea Bougainvillea sp. Red Bird of Paradise Caesalpinia pulcherrima Golden Barrel Echinocactus grusonii Red Yucca Hesperaloe parviflora Regal Mist Muhlenbergia capillaries Firecracker Penstemon Penstemon eatonii Yucca Yucca pendula Desert spoon (Grey) Dasylirion wheeleri MEDIUM WATER USE PLANT LIST Plant Type Common Name Scientific Name Trees California Fan Palm California Fan Palm California Pepper California Pepper Large Shrubs Shiny Xylosma Xylosma congestum Wax Leaf Privet Ligustrum japonicum Medium Shrubs Mexican Sage Mexican Sage Dwarf Mock Orange Dwarf Mock Orange India Hawthorne India Hawthorne Small Shrubs Weeping Bottlebrush Calistemon viminalis Mexican Bush Sage Salvia leucantha Vines Lavender Trumpet Vine Clytostoma callistegioides Queen’s Wreath Antigonon leptopus . -

Pruning Trees and Shrubs-2
Pruning Trees and Shrubs Ursula Schuch University of Arizona School of Plant Sciences 1 Publications on Pruning Pruning Deciduous Shade Trees http://extension.arizona.edu/sites/extension.arizona.edu/files/pubs/az1139.pdf Pruning Citrus http://extension.arizona.edu/sites/extension.arizona.edu/files/pubs/az1455.pdf Pruning Shrubs in the Low and Mid Elevation Deserts in Arizona http://extension.arizona.edu/sites/extension.arizona.edu/files/pubs/az1499.pdf 2 1 Pruning is the intentional removal of parts of a plant for a purpose. 3 Pruning Equipment Sterilizing tools Virus, vascular fungus, bacteria Avoid oozing cankers 70% Isopropyl alcohol Listerine, Lysol, Pine-Sol 4 2 Why Do We Prune Plants? To remove damaged/broken branches To remove rubbing, crossing, inwardly growing branches For visibility & safety considerations To train young plants Control plant size Rejuvenation of plants Increase flowering, fruiting and vigor 5 Dead branches Sign blocked Broken branch Rubbing/crossing branches 6 3 Co-dominant leader 7 Remove suckers, root stock suckers, and adventitious buds growing on the stem 8 4 Pruning Basics • Never remove more than 25‐30% of the canopy in any given year • If the plant requires frequent pruning, then it may not be the best suited plant for that situation • Usually best done in the early life of the plant • Shearing of shrubs is labor intensive, generally unnecessary, requires regular repeat shearing 9 Pruning Basics Heading versus Thinning Cut anywhere Leaves STUBS Cut back to next lateral branch NO STUBS 10 5 Where to cut? Opposite buds Above a bud when possible. Prevents unsightly stubs that die back. -

Verticillium Wilt of Fraxinus Excelsior
Verticillium wilt of Fraxinus excelsior - ' . ; Jt ""* f- "" UB-^/^'IJ::J CENTRALE LANDBOUWCATALOGUS 0000 0611 8323 locjs Promotoren: Dr.Ir. R.A.A. Oldeman Hoogleraar ind e Bosteelt en Bosoecologie Dr.Ir. J. Dekker Emeritus Hoogleraar ind e Fytopathologie /OMOS-Zöl,^ Jelle A. Hiemstra Verticillium wilt of Fraxinus excelsior Proefschrift ter verkrijging van de graad van doctor in de landbouw- en milieuwetenschappen, op gezag van de rector magnificus, dr. C.M. Karssen, inhe t openbaar te verdedigen op dinsdag 18apri l 1995 des namiddags omvie r uur ind e aula van de Landbouwuniversiteit te Wageningen J\ ABSTRACT Hiemstra, J.A. (1995). Verticillium wilt of Fraxinus excelsior. PhD Thesis, Wageningen Agricultural University, The Netherlands, xvi + 213 pp, 40 figs., 28 tables, 4 plates with colour pictures, 327 refs., English and Dutch summaries. ISBN 90-5485-360-3 Research on ash wilt disease, a common disease of Fraxinus excelsior L. in young forest and landscape plantings in several parts of the Netherlands, is described. By means of a survey for pathogenic fungi in affected trees, inoculation and reisolation experimentsi ti sdemonstrate d thatth ediseas ei scause db y Verticilliumdahliae Kleb . Hostspecificit y andvirulenc e of aV. dahliae isolatefro m ashar ecompare d tothos e of isolatesfro m elm,mapl ean dpotato .Diseas eincidenc ean dprogress , andrecover y of infected trees are investigated through monitoring experiments in two permanent plots in seriously affected forest stands. Monitoring results are related to the results of an aerial survey for ash wilt disease in the province of Flevoland to assess the impact of the disease on ash forests. -

Recovery Plan for Pimelea Spicata Pimelea Spicata Recovery Plan
© Department of Environment and Conservation (NSW), 2005 This work is copyright, however material presented in this plan may be copied for personal use or published for educational purposes, providing that any extracts are fully acknowledged. Apart from this and any other use as permitted under the Copyright Act 1968, no part may be reproduced without prior written permission from the Department of Environment and Conservation. The NPWS is part of the Department of Environment and Conservation Department of Environment and Conservation 43 Bridge Street (PO Box 1967) Hurstville NSW 2220 www.nationalparks.nsw.gov.au Requests for information or comments regarding the recovery program for Pimelea spicata should be directed to: The Director General, Department of Environment and Conservation (NSW) C/- Coordinator Pimelea spicata recovery program Biodiversity Conservation Section, Metropolitan Branch Environment Protection and Regulation Division Department of Environment and Conservation (NSW) PO Box 1967 Hurstville NSW 2220 Ph: (02) 9585 6678 Fax: (02) 9585 6442 Cover photograph: Pimelea spicata in flower growing amongst grasses at Mt Warrigal in the Illawarra Photographer: Martin Bremner This Plan should be cited as following: Department of Environment and Conservation (2005) Pimelea spicata R. Br. Recovery Plan. Department of Environment and Conservation (NSW), Hurstville NSW. ISBN: 1 74137 333 6 DEC 2006/181 Approved Recovery Plan for Pimelea spicata Pimelea spicata Recovery Plan Executive summary This document constitutes the formal Commonwealth and New South Wales State Recovery Plan for the small shrub Pimelea spicata (Thymelaeaceae), and as such considers the conservation requirements of the species across its known range. It identifies the future actions to be taken to ensure the long-term viability of P. -

Crapemyrtle Bark Scale Acanthococcus (=Eriococcus) Lagerstroemiae
Crapemyrtle Bark Scale Acanthococcus (=Eriococcus) lagerstroemiae Jim Robbins, Univ. of Ark. CES, Bugwood.org, #5521505 The crapemyrtle bark scale (Acanthococcus (=Eriococcus) lagerstroemiae) is in the family Eriococcidae (or Acanthococcidae, as the taxonomy of this family is still being debated). This group is in the superfamily Coccoidea (scale insects) and the order Hemiptera (true bugs). The primary host in North America, crapemyrtle, Lagerstroemia spp., are deciduous flowering trees popular in ornamental landscapes. They are top-sellers in the nursery trade, with the annual wholesale value estimated to be $66 million in 2014. Based on urban tree inventories of several major cities in the southeastern U.S., crapemyrtle are among the most common landscape trees planted in this region. Resources - Cai, X., H. Dou, M. Gu, M. Merchant, and E. Vafaie. 2015. Update on crapymyrtle bark scale. Proceedings of the 2015 Annual Meeting of the International Plant Propagators’ Society. 1140: 415–418. - Wang, Z., Y. Chen, M. Gu, E. Vafaie, M. Merchant, and R. Diaz. 2016. Crapemyrtle bark scale: A new threat for crapemyrtles, a popular landscape plant in the U.S. Insects. 7: 33–34. 1 Crapemyrtle Bark Scale Close-up of a crapemyrtle bark scale • In the group of insects infestation, showing the fuzzy, felt-like known as felt scales covering of the adult female scales • Native to Asia • First report in the US – 2004 in Richardson, Texas (Dallas County) • Formerly known as Eriococcus lagerstroemiae Kuwana Gary Brooks, Bayer CropScience, Bugwood.org #5523058 The crapemyrtle bark scale (abbreviated as CMBS) belongs to a group of scale insects known as felt scales or bark scales. -

Developmental Biology of Leptoypha Mutica (Hemiptera: Tingidae) on Chinese Privet (Lamiales: Oleaceae) Author(S): J
Developmental Biology of Leptoypha mutica (Hemiptera: Tingidae) on Chinese Privet (Lamiales: Oleaceae) Author(s): J. Kalina, S.K. Braman, and J.L. Hanula Source: Journal of Entomological Science, 52(2):154-160. Published By: Georgia Entomological Society https://doi.org/10.18474/JES16-28.1 URL: http://www.bioone.org/doi/full/10.18474/JES16-28.1 BioOne (www.bioone.org) is a nonprofit, online aggregation of core research in the biological, ecological, and environmental sciences. BioOne provides a sustainable online platform for over 170 journals and books published by nonprofit societies, associations, museums, institutions, and presses. Your use of this PDF, the BioOne Web site, and all posted and associated content indicates your acceptance of BioOne’s Terms of Use, available at www.bioone.org/page/terms_of_use. Usage of BioOne content is strictly limited to personal, educational, and non-commercial use. Commercial inquiries or rights and permissions requests should be directed to the individual publisher as copyright holder. BioOne sees sustainable scholarly publishing as an inherently collaborative enterprise connecting authors, nonprofit publishers, academic institutions, research libraries, and research funders in the common goal of maximizing access to critical research. Developmental Biology of Leptoypha mutica (Hemiptera: Tingidae) on Chinese Privet (Lamiales: Oleaceae)1 J. Kalina2, S.K. Braman3, and J.L. Hanula4 University of Georgia, Department of Entomology, 413 Biological Sciences Building, Athens, Georgia 30602 USA J. Entomol. Sci. 52(2): 154–160 (April 2017) Abstract The native lace bug, Leptoypha mutica Say (Hemiptera: Tingidae), has demonstrated potential as an insect biological control agent of invasive Chinese privet (Ligustrum sinense Lour). -

Yorkhill Green Spaces Wildlife Species List
Yorkhill Green Spaces Wildlife Species List April 2021 update Yorkhill Green Spaces Species list Draft list of animals, plants, fungi, mosses and lichens recorded from Yorkhill, Glasgow. Main sites: Yorkhill Park, Overnewton Park and Kelvinhaugh Park (AKA Cherry Park). Other recorded sites: bank of River Kelvin at Bunhouse Rd/ Old Dumbarton Rd, Clyde Expressway path, casual records from streets and gardens in Yorkhill. Species total: 711 Vertebrates: Amhibians:1, Birds: 57, Fish: 7, Mammals (wild): 15 Invertebrates: Amphipods: 1, Ants: 3, Bees: 26, Beetles: 21, Butterflies: 11, Caddisflies: 2, Centipedes: 3, Earthworms: 2, Earwig: 1, Flatworms: 1, Flies: 61, Grasshoppers: 1, Harvestmen: 2, Lacewings: 2, Mayflies: 2, Mites: 4, Millipedes: 3, Moths: 149, True bugs: 13, Slugs & snails: 21, Spiders: 14, Springtails: 2, Wasps: 13, Woodlice: 5 Plants: Flowering plants: 174, Ferns: 5, Grasses: 13, Horsetail: 1, Liverworts: 7, Mosses:17, Trees: 19 Fungi and lichens: Fungi: 24, Lichens: 10 Conservation Status: NameSBL - Scottish Biodiversity List Priority Species Birds of Conservation Concern - Red List, Amber List Last Common name Species Taxon Record Common toad Bufo bufo amphiban 2012 Australian landhopper Arcitalitrus dorrieni amphipod 2021 Black garden ant Lasius niger ant 2020 Red ant Myrmica rubra ant 2021 Red ant Myrmica ruginodis ant 2014 Buff-tailed bumblebee Bombus terrestris bee 2021 Garden bumblebee Bombus hortorum bee 2020 Tree bumblebee Bombus hypnorum bee 2021 Heath bumblebee Bombus jonellus bee 2020 Red-tailed bumblebee Bombus -

The Vegetation of the Western Blue Mountains Including the Capertee, Coxs, Jenolan & Gurnang Areas
Department of Environment and Conservation (NSW) The Vegetation of the Western Blue Mountains including the Capertee, Coxs, Jenolan & Gurnang Areas Volume 1: Technical Report Hawkesbury-Nepean CMA CATCHMENT MANAGEMENT AUTHORITY The Vegetation of the Western Blue Mountains (including the Capertee, Cox’s, Jenolan and Gurnang Areas) Volume 1: Technical Report (Final V1.1) Project funded by the Hawkesbury – Nepean Catchment Management Authority Information and Assessment Section Metropolitan Branch Environmental Protection and Regulation Division Department of Environment and Conservation July 2006 ACKNOWLEDGMENTS This project has been completed by the Special thanks to: Information and Assessment Section, Metropolitan Branch. The numerous land owners including State Forests of NSW who allowed access to their Section Head, Information and Assessment properties. Julie Ravallion The Department of Natural Resources, Forests NSW and Hawkesbury – Nepean CMA for Coordinator, Bioregional Data Group comments on early drafts. Daniel Connolly This report should be referenced as follows: Vegetation Project Officer DEC (2006) The Vegetation of the Western Blue Mountains. Unpublished report funded by Greg Steenbeeke the Hawkesbury – Nepean Catchment Management Authority. Department of GIS, Data Management and Database Environment and Conservation, Hurstville. Coordination Peter Ewin Photos Kylie Madden Vegetation community profile photographs by Greg Steenbeeke Greg Steenbeeke unless otherwise noted. Feature cover photo by Greg Steenbeeke. All Logistics -

Ist Β-Glucosidase Ein Schlüsselenzym Für Die
Ist β-Glucosidase ein Schlüsselenzym für die Anpassung an Iridoidglycoside? Ein Vergleich zwischen herbivoren Generalisten (Arctiidae, Lepidoptera) und einem Iridoidglycosidspezialist (Nymphalidae, Lepidoptera) Dissertation zur Erlangung der Doktorgrades der Naturwissenschaften (Dr. rer. nat.) im Department Biologie der Fakultät für Mathematik, Informatik und Naturwissenschaften an der Universität Hamburg vorgelegt von Helga Carola Pankoke aus Freiburg im Breisgau Hamburg 2010 Erstgutachterin: Professor Dr. Susanne Dobler Zweitgutachter: Professor Dr . Reinhard Lieberei Tag der Disputation: 9. April 2010 Bestätigung zur sprachlichen Korrektheit der in englischer Sprache abgefassten Dissertation Die sprachliche Korrektheit der in englischer Sprache abgefassten Dissertation von Frau Helga Carola Pankoke mit wurde von der Unterzeichnenden geprüft und bestätigt. (engl . „The grammatical and linguistical correctness of the dissertation, which has been written in English by Ms. Helga Carola Pankoke, has been proved and confirmed.“) (M. Deane Bowers) Museum and Department of Ecology and Evolutionary Biology, UCB 334 University of Colorado, Boulder Colorado 80309, USA Inhaltsverzeichnis Zusammenfassung i 1 Allgemeine Einleitung 01 2 Influence of iridoid glycoside containing host plants on midgut β-glucosidase activity in the yellow woolly bear, Spilosoma virginica (Arctiidae) 06 2.1 Summary 06 2.2 Introduction 07 2.3 Materials and Methods 10 2.4 Results 14 2.5 Discussion 19 3 The interplay between toxin-releasing β-glucosidase and plant iridoid