Benthic Communities of the Whanganui River Catchment: the Effects of Land Use and Geology
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-
Schedule D Part3
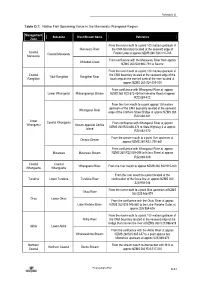
Schedule D Table D.7: Native Fish Spawning Value in the Manawatu-Wanganui Region Management Sub-zone River/Stream Name Reference Zone From the river mouth to a point 100 metres upstream of Manawatu River the CMA boundary located at the seaward edge of Coastal Coastal Manawatu Foxton Loop at approx NZMS 260 S24:010-765 Manawatu From confluence with the Manawatu River from approx Whitebait Creek NZMS 260 S24:982-791 to Source From the river mouth to a point 100 metres upstream of Coastal the CMA boundary located at the seaward edge of the Tidal Rangitikei Rangitikei River Rangitikei boat ramp on the true left bank of the river located at approx NZMS 260 S24:009-000 From confluence with Whanganui River at approx Lower Whanganui Mateongaonga Stream NZMS 260 R22:873-434 to Kaimatira Road at approx R22:889-422 From the river mouth to a point approx 100 metres upstream of the CMA boundary located at the seaward Whanganui River edge of the Cobham Street Bridge at approx NZMS 260 R22:848-381 Lower Coastal Whanganui From confluence with Whanganui River at approx Whanganui Stream opposite Corliss NZMS 260 R22:836-374 to State Highway 3 at approx Island R22:862-370 From the stream mouth to a point 1km upstream at Omapu Stream approx NZMS 260 R22: 750-441 From confluence with Whanganui River at approx Matarawa Matarawa Stream NZMS 260 R22:858-398 to Ikitara Street at approx R22:869-409 Coastal Coastal Whangaehu River From the river mouth to approx NZMS 260 S22:915-300 Whangaehu Whangaehu From the river mouth to a point located at the Turakina Lower -

Pipiriki Township
IOFFICIALI Wai 903, #A23 PIPIRIKI TOWNSHIP BRIAN HERLIHY & ASSOCIATES This report was commissioned by the Waitangi Tribunal following a claim by the Ranana Trust relating to land in the Pipiriki Township (Wai 428) September 1995 PROPRIETORS OF PIPIRIKI TOWNSHIP NO. 1 7 OTHER BLOCKS INC. (Maori Affairs Act 1953) Pipiriki Township No. 1 Whakaihuwaka C12 Waharangi 7A All correspondence to : Waharangi 7A3 The Secretary Waharangi No. 4 PO Box 282, WANGANUI Telephone/Fax 06 345 4172 20 January 1994 RECEIVED 26.1.94 The Chairman Waitangi Tribunal PO Box 5022, WELLINGTON Dear Sir The Incorporation hereby notifies a Statement of Claim in respect of two parcels of land in Pipiriki Township which it considers should be returned to Maori Ownership from The Crown. (a) Section 24 Block I - Pipiriki Township. This land was originally surveyed in the early 1990’s as a European Cemetery and at that time had three marked graves on it. Since then no further European burials have been made in Pipiriki and it is considered that the parcel of land set aside for a cemetery reserve is extremely excessive especially when compared with similar Maori burial sites in Pipiriki. (b) Sections 1, 2, 3 Block IX - Pipiriki Township. This land was designated a Crown Reserve in 1988 without reference to any persons or organisations as Owners of such land. In both cases the PROPRIETORS OF PIPIRIKI TOWNSHIP NO. 1 AND OTHER BLOCKS INC. is the correct claimant of such land being the body in whom freehold title of Pipiriki Township is vested. This claim is made under the auspices of the Whare Ranana Trust who are administering all such claims in the Pipiriki area presently with the Waitangi Tribunal. -

Counting Kākahi Connected L3 TSM.Pdf
CONNECTED, LEVEL 3 2014, Why Is That? Counting Kākahi by Hannah Rainforth Overview This is a report about research that’s been done by a scientist. Hannah investigated kākahi in the Whanganui River to find whether the evidence supports claims by local kaumātua that they are disappearing. A Google Slides version of this article is available at www.connected.tki.org.nz. Science capability: Use evidence Text characteristics Science is a way of explaining the world. Science is An informal, conversational voice that includes empirical and measurable. This means that in science, questions to the reader and rhetorical questions. explanations need to be supported by evidence that is Photographs with captions, diagrams, illustrations, based on, or derived from, observations of the natural subheadings, and a glossary. world. Students should be encouraged to support their ideas with evidence and look for evidence that supports or Complex sentences containing explanations. contradicts other explanations. Scientific and technological vocabulary and words in At the core of science is theory building – making better te reo Māori that may be unfamiliar to some students. explanations. What sets scientific explanations apart from other ways of explaining the world is their reliance on evidence and their ability to evolve as new evidence comes to light. For more information about the “Use evidence” science capability, go to http://scienceonline.tki.org.nz/Introducing- five-science-capabilities/Use-evidence Curriculum context SCIENCE NATURE OF SCIENCE: LIVING WORLD: Ecology Key Nature of Science ideas Understanding about science Achievement objective(s) Evidence is based on, or derived from, observations of the natural world. -

Water Quality Trends in the Manawatu-Wanganui Region 1989-2004
Water Quality Trends in the Manawatu-Wanganui Region 1989-2004 March 2006 Authors Ron Gibbard Royal Society of New Zealand Teacher Fellow 2005 Host: Horizons Regional Council Jon Roygard, Olivier Ausseil, Lindsay Fung Horizons Regional Council Acknowledgements Maree Clark, Horizons Regional Council Bill Vant, Environment Waikato Graeme McBride, National Institute of Water and Atmospheric Research Ltd. Russell Death, Institute of Freshwater Ecology, Massey University Some flow data in this report has been supplied by NIWA and Genesis Energy March 2006 ISBN: 1-877413-27-5 Report No: 2006/EXT/702 For more information contact Offices: Head Office: Depots: Taumarunui Palmerston North Levin 34 Maata Street 11-15 Victoria Avenue 11 Bruce Road Phone 07 895 5209 Phone 06 952 2800 Phone 06 367 8259 Wanganui Facsimile 06 952 2929 Taihape 181 Guyton Street Freephone 0508 446 749 Torere Rd, Ohotu Phone 0508 446 749 Phone 06 388 0192 [email protected] Marton Pahiatua Hammond Street www.horizons.govt.nz Cnr Huxley & Queen Streets Phone 06 327 7189 24 hr Pollution Hotline Phone 06 376 7758 Freephone 0508 476 558 Dannevirke Kairanga Weber Road Cnr Rongotea & Kairanga-Bunnythorpe Roads Phone 06 374 6700 Phone 06 350 1761 CONTENTS 1. Introduction 3 1.1 Scope of Report 3 1.2 Background to Trends Analysis Work by Horizons 3 2. Methods 5 2.1 Water Quality Sites 5 2.2 Variables 5 2.3 Datasets 6 2.4 Homogeneity of Data 6 2.5 Organising and Presenting the Datasets 6 2.6 Statistical Analyses 8 2.6.1 The Seasonal Kendall Slope Estimator (SKSE) 9 2.6.2 The Seasonal Kendall Trend Test 9 2.7 Flow Adjustment of Data 9 2.8 Flow Records for Sites 10 3. -

Sister Makareta Tawaroa
Freire at the flaxroots: analysis and action in Aotearoa Sister Makareta Tawaroa Te Rongo o te Poi te whare karakia Taritariwhioi te whenua Kaiwhaiki te marae Nga Paerangi te iwi Whanganui te awa Tihei Mauriora My name is Takahia Makareta Tawaroa. I was born and bred at Taritariwhioi, Kaiwhaiki Pa on the lower reaches of the Whanganui River in a small, close-knit community of about 200 people. There were five in our family, four girls and one boy. My mother was Raina Kahukura, a warm, handsome woman who was steeped in the knowledge of our old people and their ways and was a great singer and performer. Mum was also a great orator and a keen compeEtor at the Hui Aranga. For many years she competed in the Senior Oratory and achieved a lot of success. My father was Te Kohiroa Tawaroa Tetana a hardworking man who spent hours in the garden, planEng, weeding and producing acres of kai, Maori potato, Maori corn, kamokamo, kumara and pumpkin. One of my earliest memories is of planEng rows and rows of corn alongside him. In 1965 at the age of 20, I joined the Congregaon of the Sisters of St Joseph of Nazareth. I had a strong Catholic upbringing and mum was a devout churchgoer. Our family recited the Rosary most nights and we aended Mass on Sundays. I received catechism lessons from Father Cook who came to Upokongaro School every Friday from St Mary’s parish. He would bring his tuning fork because he loved to hear us sing. His favourite hymn was Mo Maria. -

Taranaki/Wanganui Conservation Board
TARANAKI / WHANGANUI CONSERVATION BOARD TE TAI HAU-Ā-URU Private Bag 3016 WANGANUI Telephone: (06) 3488475 Facsimile: (06) 3488095 e-mail: [email protected] ANNUAL REPORT FOR YEAR ENDED 30 JUNE 2006 Presented to the NZ Conservation Authority pursuant to Section 6 (O) of the Conservation Act 1987 ISSN: 1171-2236 DOCDM 40046 SBC10/6 1. Introduction to Board Members The following members were appointed by the Minister of Conservation to the Taranaki/Whanganui Conservation Board. Chairperson: Karen Schumacher, Appointed 2002 -Taranaki Deputy Chairperson: Jenny Steven, Appointed 2004 –Taranaki Board Members: Ann Abernethy, Appointed 2003 -Taihape Barney Haami, Appointed 1998 - Wanganui Stephen Sammons, Appointed 2002 - Wanganui Jonathan Procter, Appointed 2003 - Palmerston North Christine Cheyne, Appointed 2004 - Palmerston North Jenny Steven, Appointed 2004 – Taranaki Janet Gray, Appointed 2004 – Taranaki Huirangi Waikerepuru, Reappointed 2005 – Taranaki Darryn Ratana, Appointed 2005 - Wanganui Dave Digby, Appointed May 2006 (to replace Janet Gray) – Taranaki Ian Bell, Appointed 2005 – Wanganui Rachel Steele, Appointed 2005 – Taumarunui Barbara Arnold, Reappointed 2002 – Palmerston North Board members who Janet Gray, Resigned October 2005 retired/resigned during Barbara Arnold, Retired, September 2005 the period covered Stephen Sammons, Resigned June 2006 by this Report The board did not co-opt any members during the report period. 2. Functions and Powers of the Board The board has the responsibility for shaping conservation management through advising the department on the development of the Conservation Management Strategy (CMS) for the Wanganui Conservancy and Conservation Management Plans (CMPs) for public conservation lands whereas the Department of Conservation is responsible for the day to day management of these areas. -

Te Korowai O Wainuiārua Historical Account – Draft for Eminent Historian Review Contents
Te Korowai o Wainuiārua historical account – 25 June 2020 Te Korowai o Wainuiārua Historical Account – Draft for eminent historian review Contents Te Korowai o Wainuiārua Historical Account – Draft for eminent historian review .............................. 1 1. Uenuku, Tamakana, and Tamahaki at 1840 and the Treaty of Waitangi ....................................... 3 Uenuku, Tamakana, and Tamahaki identity at 1840 .......................................................................... 3 Uenuku ............................................................................................................................................ 3 Tamakana ........................................................................................................................................ 3 Tamahaki ......................................................................................................................................... 3 The Treaty of Waitangi........................................................................................................................ 4 2. Uenuku, Tamakana, and Tamahaki and the Colonial State 1840-1860 .......................................... 4 The New Zealand Company ................................................................................................................ 4 The Spain Commission ........................................................................................................................ 5 Crown purchasing negotiations ......................................................................................................... -

Whanganui River Canoe Itinerary
WHANGANUI RIVER CANOE ITINERARY Paddle right through the Whanganui National Park, a remote and impenetrable region rich in Māoritanga (Maori culture). Deep jungles and incised rivers extend for 700 square kilometres and there is almost no road access. Canadian canoes are a comfortable and classic choice for this journey. Explore and enjoy the mighty Whanganui River - the life force of the area as it weaves its way from the mountains to the sea. LENGTH 5 days GRADE B (“Soft Adventure” with an element of challenge) * PRE-TRACK BRIEFING: Please call by the base at 6 Tay Street, Ohakune, the day before to collect your dry bags for packing. START 6:45am, 6 Tay St, Ohakune FINISH 3:30pm, Ohakune DEPARTURES October 2021: 4, 11, 18, 25 November 2021: 1, 8, 15, 22, 29 December 2021: 6, 13, 20, 27 January 2022: 3, 10, 17, 24, 31 February 2022: 7, 14, 21, 28 March 2022: 7, 14, 21, 28 April 2022: 4, 11, 18, 25 May 2022: 2, 9, 16, 23, 30 PRICE 1st Jun 2021 – 30th Jun 2022 5 Day Adult NZ$1345 5 Day Child NZ$1095 4 Day Adult NZ$1145 4 Day Child NZ$995 5-day trips depart on Mondays (dates above). 4-day trips depart the following date (on Tuesdays), to book the 4 day trip please enquire. The 4-day trip begins at 7.15am and follows Days 2 - 5 of the 5-day itinerary. “WE COULDN’T HAVE WANTED FOR ANYONE “5 DAYS OF ADVENTURE & FUN OF RIVER” BETTER” My 18 yr old son and I had a fantastic time on our trip, 5 days canoeing the A wonderful five day trip down the river in perfect weather. -

WHANGANUI DISTRICT MARAE INFORMATION Register Item No: 5 WDC Property: 18029
WHANGANUI DISTRICT MARAE INFORMATION Register Item No: 5 WDC Property: 18029 Marae Name: Rānana Marae Hapū: Ngāti Ruaka, Ngāti Hine, Ngāti Rangi Wharenui: Te Morehu Wharekai: Ruaka Hall Location: 4475 Whanganui River Road, Ranana, Whanganui Legal Description: Rānana Reserve Part 10, Block VI Tauakira S.D, Whanganui Contact Details: Rāwiri Tinirau – Chairperson [email protected] Trustees: Evelyn Broad, Tamatete Jackson, Brendon Te Tiwha James Puketapu, Terrence James Tapa. Rānana Marae (also known as Ruakā) is in Ranana, along the Whanganui River. Its principal hapū is Ngāti Ruakā of the iwi Te Ātihaunui a Pāpārangi. The wharenui is named Te Morehu, and the wharekai is Ruaka Hall. The marae connects ancestrally to the waka Aotea, the maunga Ruapehu and the awa Whanganui. Whare: Te Morehu Wharekai: Ruaka Hall Hapū: Ngāti Ruaka, Ngāti Hine, Ngāti Rangi Waka: Aotea Maunga: Ruapehu Awa: Whanganui Rohe: Te Tai Hauāuru Iwi: Te Ātihaunui a Pāpārangi Section 5 Block VI Tauakira Survey District and Part Ranana Reserve 10 Block & Part Ranana Reserve 10 Block Title Details Report - Block 19861 Block ID : 19861 Land Status : Maori Freehold Land District : Aotea Plan : ML 3811 Title Order Type: Partition Order LINZ Ref: WN24C/981, 484473 Title Order Ref: 106 WG 59-60 Area (ha): 5.8159 Title Notice Ref: - Total Shares: 30.5 Title Order Date: 18/12/1923 Total Owners: 190 Sourced from Maori Land Online (www.maorilandonline.govt.nz) - Crown Copyright Reserved. Disclaimer: The information contained in this report is a summary only - the accuracy of the Maori Land Court record, is itself, not accompanied by a state guarantee and to ensure accuracy users will need to verify the information against the records held by the Maori Land Court and the corresponding title information issued by Land Information New Zealand. -

ENVIRONMENTAL REPORT // 01.07.11 // 30.06.12 Matters Directly Withinterested Parties
ENVIRONMENTAL REPORT // 01.07.11 // 30.06.12 2 1 This report provides a summary of key environmental outcomes developed through the process to renew resource consents for the ongoing operation of the Tongariro Power Scheme. The process to renew resource consents was lengthy and complicated, with a vast amount of technical information collected. It is not the intention of this report to reproduce or replicate this information in any way, rather it summarises the key outcomes for the operating period 1 July 2011 to 30 June 2012. The report also provides a summary of key result areas. There are a number of technical reports, research programmes, environmental initiatives and agreements that have fed into this report. As stated above, it is not the intention of this report to reproduce or replicate this information, rather to provide a summary of it. Genesis Energy is happy to provide further details or technical reports or discuss matters directly with interested parties. HIGHLIGHTS 1 July 2011 to 30 June 2012 02 01 INTRODUCTION 02 1.1 Document Overview Rotoaira Tuna Wananga Genesis Energy was approached by 02 1.2 Resource Consents Process Overview members of Ngati Hikairo ki Tongariro during the reporting period 02 1.3 How to use this document with a proposal to the stranding of tuna (eels) at the Wairehu Drum 02 1.4 Genesis Energy’s Approach Screens at the outlet to Lake Otamangakau. A tuna wananga was to Environmental Management held at Otukou Marae in May 2012 to discuss the wider issues of tuna 02 1.4.1 Genesis Energy’s Values 03 1.4.2 Environmental Management System management and to develop skills in-house to undertake a monitoring 03 1.4.3 Resource Consents Management System and management programme (see Section 6.1.3 for details). -

COVID-19 Community Experience Report
COVID 19 COMMUNITY LIVED EXPERIENCE REPORT Reflections from members of the communities of Rangitikei, Ruapehu, Whanganui and South Taranaki 1 ABSTRACT There wasn’t just one lockdown – we all had our own experience. This report presents the lived experiences of the COVID-19 Level 4 lockdown for members of the Whanganui, Ruapehu, Rangitikei and South Taranaki regions. It serves to provide insights to members of the Impact Collective and our communities to our sense of wellbeing during our collective experience of lockdown. Disclaimer: As a result of the narratives being presented verbatim, some people may find the language and images contained within this report offensive or objectionable. Reader discretion is advised. Report Author Steve Carey 2 ACKNOWLEDGEMENTS The team would like to thank the following organisations and representatives for taking the time to share your experiences of COVID-19 with the team. We have not listed the individuals who spoke to us 1-1 in this acknowledgement list for the purposes of anonymity. 60s Up Club MediaWorks Whanganui AccessAbility MedLab Whanganui Age Concern Mental Health and Wellbeing Support Alzheimer’s Whanganui Ministry of Social Development Aramoho Health Centre Mokai Patea Balance Whanganui National Council of Women Birthright Whanganui National Hauora Coalition Brain Injury Association Ngā Rauru-kitahi Born and Raised Pasifika Ngā Wairiki Ngāti Apa Bulls Community Group Ngāti Rangi Bulls Medical Centre New Zealand Police Bulls RSA Pathways Bulls Rural Women’s Network Positive Aging Forum Cancer -

Official Records of Central and Local Government Agencies
Wai 2358, #A87 Wai 903, #A36 Crown Impacts on Customary Maori Authority over the Coast, Inland Waterways (other than the Whanganui River) and associated mahinga kai in the Whanganui Inquiry District Cathy Marr June 2003 Table of contents Acknowledgements .................................................................................................................... 1 Introduction ................................................................................................................................ 2 Figure 1: Area covered by this Report with Selected Natural Features ................................ 7 Chapter 1 Whanganui inland waterways, coast and associated mahinga kai pre 1839 .............. 8 Introduction ............................................................................................................................ 8 1.1 The Whanganui coast and inland waterways ................................................................. 8 Figure 2: Waterways and Coast: Whanganui Coastal District ............................................. 9 1.2 Traditional Maori authority over the Whanganui environment... ................................. 20 1.3 Early contact ............................................................................................................. 31 Conclusion ............................................................................................................................ 37 Chapter 2 The impact of the Whanganui purchase 1839-1860s ............................................... 39