Conotoxin GIIIA in the Inhibition of Nav1.4
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Animal Venom Derived Toxins Are Novel Analgesics for Treatment Of
Short Communication iMedPub Journals 2018 www.imedpub.com Journal of Molecular Sciences Vol.2 No.1:6 Animal Venom Derived Toxins are Novel Upadhyay RK* Analgesics for Treatment of Arthritis Department of Zoology, DDU Gorakhpur University, Gorakhpur, UP, India Abstract *Corresponding authors: Ravi Kant Upadhyay Present review article explains use of animal venom derived toxins as analgesics of the treatment of chronic pain and inflammation occurs in arthritis. It is a [email protected] progressive degenerative joint disease that put major impact on joint function and quality of life. Patients face prolonged inappropriate inflammatory responses and bone erosion. Longer persistent chronic pain is a complex and debilitating Department of Zoology, DDU Gorakhpur condition associated with a large personal, mental, physical and socioeconomic University, Gorakhpur, UttarPradesh, India. burden. However, for mitigation of inflammation and sever pain in joints synthetic analgesics are used to provide quick relief from pain but they impose many long Tel: 9838448495 term side effects. Venom toxins showed high affinity to voltage gated channels, and pain receptors. These are strong inhibitors of ion channels which enable them as potential therapeutic agents for the treatment of pain. Present article Citation: Upadhyay RK (2018) Animal Venom emphasizes development of a new class of analgesic agents in form of venom Derived Toxins are Novel Analgesics for derived toxins for the treatment of arthritis. Treatment of Arthritis. J Mol Sci. Vol.2 No.1:6 Keywords: Analgesics; Venom toxins; Ion channels; Channel inhibitors; Pain; Inflammation Received: February 04, 2018; Accepted: March 12, 2018; Published: March 19, 2018 Introduction such as the back, spine, and pelvis. -

Mapping of Maurotoxin Binding Sites on Hkv1.2, Hkv1.3, and Hikca1 Channels
0026-895X/04/6605-1103–1112$20.00 MOLECULAR PHARMACOLOGY Vol. 66, No. 5 Copyright © 2004 The American Society for Pharmacology and Experimental Therapeutics 2774/1178283 Mol Pharmacol 66:1103–1112, 2004 Printed in U.S.A. Mapping of Maurotoxin Binding Sites on hKv1.2, hKv1.3, and hIKCa1 Channels Violeta Visan, Ziad Fajloun, Jean-Marc Sabatier, and Stephan Grissmer Department of Applied Physiology, University of Ulm, Ulm, Germany (V.V., S.G.); Laboratoire International Associe´ d’Inge´nierie Biomole´culaire, Centre National de la Recherche Scientifique Unite´ Mixte Recherche 6560, Marseille, France (Z.F., J.-M.S.) Received May 17, 2004; accepted July 27, 2004 Downloaded from ABSTRACT Maurotoxin (MTX) is a potent blocker of human voltage-acti- interactions. Lys23 from MTX protrudes into the channel pore vated Kv1.2 and intermediate-conductance calcium-activated interacting with the GYGD motif, whereas Tyr32 and Lys7 inter- potassium channels, hIKCa1. Because its blocking affinity on act with Val381, Asp363, and Glu355, stabilizing the toxin onto the both channels is similar, although the pore region of these channel pore. Because only Val381, Asp363, and the GYGD motif channels show only few conserved amino acids, we aimed to are conserved in hIKCa1 channels, and the replacement of His399 characterize the binding sites of MTX in these channels. Inves- from hKv1.3 channels with a threonine makes this channel MTX- molpharm.aspetjournals.org tigating the pHo dependence of MTX block on current through sensitive, we concluded that MTX binds to all three channels 355 hKv1.2 channels, we concluded that the block is less pHo- through the same amino acids. -
![Distribution and Kinetics of the Kv1.3-Blocking Peptide Hstx1[R14A]](https://docslib.b-cdn.net/cover/3104/distribution-and-kinetics-of-the-kv1-3-blocking-peptide-hstx1-r14a-2183104.webp)
Distribution and Kinetics of the Kv1.3-Blocking Peptide Hstx1[R14A]
www.nature.com/scientificreports OPEN Distribution and kinetics of the Kv1.3-blocking peptide HsTX1[R14A] in experimental rats Received: 19 January 2017 Ralf Bergmann1, Manja Kubeil1,2, Kristof Zarschler1, Sandeep Chhabra3, Rajeev B. Tajhya4, Accepted: 9 May 2017 Christine Beeton 4, Michael W. Pennington5, Michael Bachmann1, Raymond S. Norton 3 & Published: xx xx xxxx Holger Stephan 1 The peptide HsTX1[R14A] is a potent and selective blocker of the voltage-gated potassium channel Kv1.3, which is a highly promising target for the treatment of autoimmune diseases and other conditions. In order to assess the biodistribution of this peptide, it was conjugated with NOTA and radiolabelled with copper-64. [64Cu]Cu-NOTA-HsTX1[R14A] was synthesised in high radiochemical purity and yield. The radiotracer was evaluated in vitro and in vivo. The biodistribution and PET studies after intravenous and subcutaneous injections showed similar patterns and kinetics. The hydrophilic peptide was rapidly distributed, showed low accumulation in most of the organs and tissues, and demonstrated high molecular stability in vitro and in vivo. The most prominent accumulation occurred in the epiphyseal plates of trabecular bones. The high stability and bioavailability, low normal-tissue uptake of [64Cu]Cu-NOTA-HsTX1[R14A], and accumulation in regions of up-regulated Kv channels both in vitro and in vivo demonstrate that HsTX1[R14A] represents a valuable lead for conditions treatable by blockade of the voltage-gated potassium channel Kv1.3. The pharmacokinetics shows that both intravenous and subcutaneous applications are viable routes for the delivery of this potent peptide. Voltage-gated potassium (Kv) channels are integral membrane proteins that regulate cell membrane potential and are involved in a variety of cellular functions including apoptosis and cell volume regulation1. -

Modeling of the Binding of Peptide Blockers to Voltage-Gated Potassium Channels: Approaches and Evidence
REVIEWS Modeling of the Binding of Peptide Blockers to Voltage-Gated Potassium Channels: Approaches and Evidence V. N. Novoseletsky1*, A. D. Volyntseva1, K. V. Shaitan1, M. P. Kirpichnikov1,2, A. V. Feofanov1,2 1M.V.Lomonosov Moscow State University, Faculty of Biology, Leninskie Gory 1, bldg. 12, 119992, Moscow, Russia 2Shemyakin-Ovchinnikov Institute of Bioorganic Chemistry, Russian Academy of Sciences, Miklukho- Maklaya str. 16/10, 117997, Moscow, Russia *Email: [email protected] Received: 06.07.2015 Copyright © 2016 Park-media, Ltd. This is an open access article distributed under the Creative Commons Attribution License,which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited. ABSTRACT Modeling of the structure of voltage-gated potassium (KV) channels bound to peptide blockers aims to identify the key amino acid residues dictating affinity and provide insights into the toxin-channel interface. Computational approaches open up possibilities for in silico rational design of selective blockers, new molecular tools to study the cellular distribution and functional roles of potassium channels. It is anticipated that optimized blockers will advance the development of drugs that reduce over activation of potassium channels and attenuate the associated malfunction. Starting with an overview of the recent advances in computational simulation strat- egies to predict the bound state orientations of peptide pore blockers relative to KV-channels, we go on to review algorithms -

A Four-Disulphide-Bridged Toxin, with High Affinity Towards Voltage-Gated
Biochem. J. (1997) 328, 321–327 (Printed in Great Britain) 321 A four-disulphide-bridged toxin, with high affinity towards voltage-gated K+ channels, isolated from Heterometrus spinnifer (Scorpionidae) venom ! Bruno LEBRUN*1,Regine ROMI-LEBRUN*, Marie-France MARTIN-EAUCLAIRE†, Akikazu YASUDA*, Masaji ISHIGURO*, Yoshiaki OYAMA‡, Olaf PONGS§ and Terumi NAKAJIMA* *Suntory Institute for Bioorganic Research, Mishima-Gun, Shimamoto-Cho, Wakayamadai 1-1-1, 618 Osaka, Japan, †Laboratoire de Biochimie, CNRS UMR 6560, Faculte! de Me! decine Nord, 13916 Marseille Cedex 20, France, ‡Suntory Ltd Institute for Biomedical Research, Mishima-Gun, Shimamoto-Cho, Wakayamadai 1-1-1, 618 Osaka, Japan, and §Zentrum fu$ r Molekulare Neurobiologie, Institute fu$ r Neurale Signalverarbeitung, D-20246 Hamburg, Federal Republic of Germany A new toxin, named HsTX1, has been identified in the venom of limited reduction–alkylation at acidic pH and (2) enzymic Heterometrus spinnifer (Scorpionidae), on the basis of its ability cleavage on an immobilized trypsin cartridge, both followed by to block the rat Kv1.3 channels expressed in Xenopus oocytes. mass and sequence analyses. Three of the disulphide bonds are HsTX1 has been purified and characterized as a 34-residue connected as in the three-disulphide-bridged scorpion toxins, peptide reticulated by four disulphide bridges. HsTX1 shares and the two extra half-cystine residues of HsTX1 are cross- 53% and 59% sequence identity with Pandinus imperator toxin1 linked, as in Pi1. These results, together with those of CD (Pi1) and maurotoxin, two recently isolated four-disulphide- analysis, suggest that HsTX1 probably adopts the same general bridged toxins, whereas it is only 32–47% identical with the folding as all scorpion K+ channel toxins. -

Identification of Aethina Tumida Kir Channels As Putative Targets of The
toxins Article Identification of Aethina tumida Kir Channels as Putative Targets of the Bee Venom Peptide Tertiapin Using Structure-Based Virtual Screening Methods Craig A. Doupnik Department of Molecular Pharmacology & Physiology, University of South Florida College of Medicine, Tampa, FL 33612, USA; [email protected] Received: 20 August 2019; Accepted: 18 September 2019; Published: 19 September 2019 Abstract: Venoms are comprised of diverse mixtures of proteins, peptides, and small molecules. Identifying individual venom components and their target(s) with mechanism of action is now attainable to understand comprehensively the effectiveness of venom cocktails and how they collectively function in the defense and predation of an organism. Here, structure-based computational methods were used with bioinformatics tools to screen and identify potential biological targets of tertiapin (TPN), a venom peptide from Apis mellifera (European honey bee). The small hive beetle (Aethina tumida (A. tumida)) is a natural predator of the honey bee colony and was found to possess multiple inwardly rectifying K+ (Kir) channel subunit genes from a genomic BLAST search analysis. Structure-based virtual screening of homology modelled A. tumida Kir (atKir) channels found TPN to interact with a docking profile and interface “footprint” equivalent to known TPN-sensitive mammalian Kir channels. The results support the hypothesis that atKir channels, and perhaps other insect Kir channels, are natural biological targets of TPN that help defend the bee colony from infestations by blocking K+ transport via atKir channels. From these in silico findings, this hypothesis can now be subsequently tested in vitro by validating atKir channel block as well as in vivo TPN toxicity towards A. -
Stichodactyla Toxin Shk - #08SHK001 - CAS : 165168-50-3
Stichodactyla toxin ShK - #08SHK001 - CAS : 165168-50-3 Product information Animal toxins for life-sciences Selective blocker of Kv1.3 www.smartox-biotech.com ShK (Stichodactyla helianthus Neurotoxin) has been isolated from the venom of the Phone : +33(0) 456 520 869 Carribean sea anemone Stoichactis helianthus. ShK inhibits voltage-dependent potassium Fax : +33(0) 456 520 868 channels. It blocks Kv1.3 (KCNA3) potently and also Kv1.1 (KCNA1), Kv1.4 (KCNA4) and [email protected] Kv1.6 (KCNA6) respectively with a Kd of 11 pM, 16 pM, 312 pM and 165 pM. Interestingly, Smartox Biotechnology it was also demonstrated that ShK potently inhibits the hKv3.2b channel with an IC50 value of approximately 0.6 nM. 570 rue de la chimie 38400 Saint Martin d’Hères France Fluorescent ShK also available: TMR-ShK - #SAT001 Prices 5x10 µg – 100 € 100 µg – 120 € 500 µg – 375 € Technical information AA sequence: Arg-Ser-Cys3-Ile-Asp-Thr-Ile-Pro-Lys-Ser-Arg-Cys12-Thr-Ala-Phe-Gln-Cys17- 28 32 35 Related products Lys-His-Ser-Met-Lys-Tyr-Arg-Leu-Ser-Phe-Cys -Arg-Lys-Thr-Cys -Gly-Thr-Cys -OH 3 35 12 28 17 32 Disulfide bridges: Cys -Cys , Cys -Cys and Cys -Cys TMR-ShK - # SAT001 Length (aa): 35 Solubility: water and saline buffer Margatoxin - #08MAG001 Formula: C169H274N54O48S7 CAS number: 165168-50-3 HsTx1 - # 08NEU001 Molecular Weight: 4054.85 Da Source: Synthetic (Dap22)-ShK - # 13SHD001 Appearance: White lyophilized solid Purity rate: > 97 % ADWX-1 - # 13ADW001 Agitoxin-2 - # 13AGI002 References Tarcha EJ., et al.. - J Pharmacol Exp Ther. -

Toxins Targeting the KV1.3 Channel: Potential Immunomodulators for Autoimmune Diseases
Toxins 2015, 7, 1749-1764; doi:10.3390/toxins7051749 OPEN ACCESS toxins ISSN 2072-6651 www.mdpi.com/journal/toxins Article Toxins Targeting the KV1.3 Channel: Potential Immunomodulators for Autoimmune Diseases Yipeng Zhao 1, Jie Huang 1, Xiaolu Yuan 1, Biwen Peng 2, Wanhong Liu 3, Song Han 1,* and Xiaohua He 1,* 1 Department of Pathophysiology, School of Basic Medical Sciences, Wuhan University, Wuhan 430072, China; E-Mails: [email protected] (Y.Z.); [email protected] (J.H.); [email protected] (X.Y.) 2 Hubei Provincial Key Laboratory of Developmentally Originated Disease, School of Basic Medical Sciences, Wuhan University, Wuhan 430072, China; E-Mail: [email protected] 3 Hubei Province Key Laboratory of Allergy and Immunology, School of Basic Medical Sciences, Wuhan University, Wuhan 430072, China; E-Mail: [email protected] * Authors to whom correspondence should be addressed; E-Mails: [email protected] (S.H.); [email protected] (X.H.); Tel./Fax: +86-27-6875-9991. Academic Editor: Azzam A. Maghazachi Received: 10 April 2015 / Accepted: 5 May 2015 / Published: 19 May 2015 Abstract: Autoimmune diseases are usually accompanied by tissue injury caused by autoantigen-specific T-cells. KV1.3 channels participate in modulating calcium signaling to induce T-cell proliferation, immune activation and cytokine production. Effector memory T (TEM)-cells, which play major roles in many autoimmune diseases, are controlled by blocking KV1.3 channels on the membrane. Toxins derived from animal venoms have been found to selectively target a variety of ion channels, including KV1.3. By blocking the KV1.3 channel, these toxins are able to suppress the activation and proliferation of TEM cells and may improve TEM cell-mediated autoimmune diseases, such as multiple sclerosis and type I diabetes mellitus. -

A Potent and Kv1.3-Selective Analogue of the Scorpion Toxin
OPEN A potent and Kv1.3-selective analogue of SUBJECT AREAS: the scorpion toxin HsTX1 as a potential PEPTIDES DRUG DEVELOPMENT therapeutic for autoimmune diseases M. Harunur Rashid1,2, Redwan Huq3,4, Mark R. Tanner3,5, Sandeep Chhabra1, Keith K. Khoo1, Received Rosendo Estrada6, Vikas Dhawan6, Satendra Chauhan6, Michael W. Pennington6, Christine Beeton3, 24 December 2013 Serdar Kuyucak2 & Raymond S. Norton1 Accepted 12 March 2014 1Medicinal Chemistry, Monash Institute of Pharmaceutical Sciences, Monash University, Parkville, Victoria 3052Australia, 2School 3 Published of Physics, University of Sydney, New South Wales 2006, Australia, Department of Molecular Physiology and Biophysics, Baylor 4 28 March 2014 College of Medicine, Houston, TX 77030, USA, Graduate Program in Molecular Physiology and Biophysics, Baylor College of Medicine, Houston, TX 77030, USA, 5Interdepartmental Graduate Program in Translational Biology and Molecular Medicine, Baylor College of Medicine, Houston, TX 77030, USA, 6Peptides International, 11621 Electron Drive, Louisville, KY 40299, USA. Correspondence and requests for materials HsTX1 toxin, from the scorpion Heterometrus spinnifer, is a 34-residue, C-terminally amidated peptide should be addressed to cross-linked by four disulfide bridges. Here we describe new HsTX1 analogues with an Ala, Phe, Val or Abu S.K. (serdar@physics. substitution at position 14. Complexes of HsTX1 with the voltage-gated potassium channels Kv1.3 and usyd.edu.au) or R.S.N. Kv1.1 were created using docking and molecular dynamics simulations, then umbrella sampling simulations were performed to construct the potential of mean force (PMF) of the ligand and calculate the (ray.norton@monash. corresponding binding free energy for the most stable configuration. -

The 'Functional' Dyad of Scorpion Toxin Pi1 Is Not Itself a Prerequisite For
Biochem. J. (2004) 377, 25–36 (Printed in Great Britain) 25 The ‘functional’ dyad of scorpion toxin Pi1 is not itself a prerequisite for toxin binding to the voltage-gated Kv1.2 potassium channels Stephanie´ MOUHAT*†, Amor MOSBAH*†, Violeta VISAN‡, Heike WULFF§, Muriel DELEPIERRE¶,Herve´ DARBON, Stephan GRISSMER‡, Michel DE WAARD** and Jean-Marc SABATIER*†1 *Laboratoire International Associed’Ing´ enierie´ Biomoleculaire,´ Boulevard Pierre Dramard, 13916 Marseille Cedex 20, France, †Laboratoire de Biochimie, CNRS UMR 6560, Boulevard Pierre Dramard, 13916 Marseille Cedex 20, France, ‡Universitat¨ Ulm, Albert Einstein Allee 11, 89081 Ulm, Germany, §Department of Physiology and Biophysics, University of California, Irvine, CA 92697, U.S.A., ¶Laboratoire de RMN, Institut Pasteur, CNRS URA 1129, 28 Rue du Dr. Roux, 75724, Paris, France, AFMB, CNRS UPR 9039, 31 Chemin Joseph Aiguier, 13402 Marseille, France, and **Inserm EMI 9931, 17 Rue des Martyrs, 38054 Grenoble Cedex 09, France Pi1 is a 35-residue scorpion toxin cross-linked by four disulphide SK channels of rat brain synaptosomes (IC50 values of 30 and bridges that acts potently on both small-conductance Ca2+- 10 nM, respectively) and block rat voltage-gated Kv1.2 channels + activated (SK) and voltage-gated (Kv) K channel subtypes. Two expressed in Xenopus laevis oocytes (IC50 values of 22 µMand approaches were used to investigate the relative contribution 75 nM, respectively), whereas they are inactive on Kv1.1 or Kv1.3 of the Pi1 functional dyad (Tyr-33 and Lys-24) to the toxin channels at micromolar concentrations. Therefore, although both action: (i) the chemical synthesis of a [A24,A33]-Pi1 analogue, analogues are less active than Pi1 both in vivo and in vitro,the lacking the functional dyad, and (ii) the production of a Pi1 integrity of the Pi1 functional dyad does not appear to be a analogue that is phosphorylated on Tyr-33 (P-Pi1). -
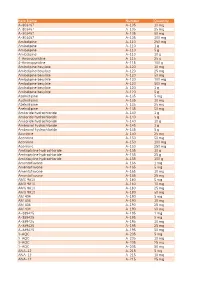
Item Name Catalog Number Quantity A-803467 A-105 10 Mg A
Catalog Item Name Number Quantity A-803467 A-105 10 mg A-803467 A-105 25 mg A-803467 A-105 50 mg A-803467 A-105 100 mg Amlodipine A-110 250 mg Amlodipine A-110 1 g Amlodipine A-110 5 g Amlodipine A-110 10 g 4-Aminopyridine A-115 25 g 4-Aminopyridine A-115 100 g Amlodipine besylate A-120 10 mg Amlodipine besylate A-120 25 mg Amlodipine besylate A-120 50 mg Amlodipine besylate A-120 100 mg Amlodipine besylate A-120 500 mg Amlodipine besylate A-120 1 g Amlodipine besylate A-120 5 g Azelnidipine A-135 5 mg Azelnidipine A-135 10 mg Azelnidipine A-135 25 mg Azelnidipine A-135 50 mg Amiloride hydrochloride A-140 1 g Amiloride hydrochloride A-140 5 g Amiloride hydrochloride A-140 10 g Ambroxol hydrochloride A-145 1 g Ambroxol hydrochloride A-145 5 g Aconitine A-150 25 mg Aconitine A-150 50 mg Aconitine A-150 100 mg Aconitine A-150 250 mg Amitriptyline hydrochloride A-155 10 g Amitriptyline hydrochloride A-155 25 g Amitriptyline hydrochloride A-155 100 g Amentoflavone A-165 1 mg Amentoflavone A-165 5 mg Amentoflavone A-165 10 mg Amentoflavone A-165 25 mg AMG 9810 A-180 5 mg AMG 9810 A-180 10 mg AMG 9810 A-180 25 mg AMG 9810 A-180 50 mg AM 404 A-190 5 mg AM 404 A-190 10 mg AM 404 A-190 25 mg AM 404 A-190 50 mg A-889425 A-195 1 mg A-889425 A-195 5 mg A-889425 A-195 10 mg A-889425 A-195 25 mg A-889425 A-195 50 mg 3-AQC A-205 5 mg 3-AQC A-205 10 mg 3-AQC A-205 25 mg 3-AQC A-205 50 mg ANA-12 A-215 5 mg ANA-12 A-215 10 mg ANA-12 A-215 25 mg ANA-12 A-215 50 mg ANA-12 A-215 100 mg A 967079 A-225 5 mg A 967079 A-225 10 mg A 967079 A-225 25 mg A 967079 -

Structural and Functional Study of Potassium Channel Inhibitor Hstx1
Structural and functional study of potassium channel inhibitor HsTX1 Mao-Feng Ger Introduction Membrane proteins are thought to account for 30% of genes; thus there may be at least 10 000 membrane proteins encoded in the human genome. Ion channels are integral membrane proteins that allow movement of ions across membranes down their electro- chemical gradients. These channel proteins form water-filled, gated pores that are often highly selective for specific ions (such as sodium, calcium, potassium or chloride). Only when the channels are open, ions can flow in and out. The opening of ion channels depends on of membrane potential (voltage-gated channels), binding of signaling molecules such as neurotransmitters, ions or nucleotides (ligand-gated channels) or stretch of the membrane (mechanosensitive channels). Potassium channels comprise a large family of ion channels. Their physiological functions are quite important, concerned with maintaining a negative voltage inside cells relative to outside. K channels have different families, according to their gating mechanism, i.e. the control of opening and closing of the channel. All K channels share the same core structure. Voltage-gated potassium channel is made from 4 alpha polypeptides forming a central pore. Each polypeptide has 6 transmembrane regions. The beta chain is regulatory and can interact with the alpha subunit to regulate the gating kinetics and enhance the stability of the complex. Another class of potassium channels is in-ward rectifiers. The channel is made from 4 identical subunits. They have 2 membrane-spanning segments and 1 pore-lining segment. Analysis of K channel sequences can understand the relationships between sequence motifs and various aspects of physiological function.