Chemical and Microbial Characterization of Ropy Maple Sap
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Pentose PO4 Pathway, Fructose, Galactose Metabolism.Pptx
Pentose PO4 pathway, Fructose, galactose metabolism The Entner Doudoroff pathway begins with hexokinase producing Glucose 6 PO4 , but produce only one ATP. This pathway prevalent in anaerobes such as Pseudomonas, they doe not have a Phosphofructokinase. The pentose phosphate pathway (also called the phosphogluconate pathway and the hexose monophosphate shunt) is a biochemical pathway parallel to glycolysis that generates NADPH and pentoses. While it does involve oxidation of glucose, its primary role is anabolic rather than catabolic. There are two distinct phases in the pathway. The first is the oxidative phase, in which NADPH is generated, and the second is the non-oxidative synthesis of 5-carbon sugars. For most organisms, the pentose phosphate pathway takes place in the cytosol. For each mole of glucose 6 PO4 metabolized to ribulose 5 PO4, 2 moles of NADPH are produced. 6-Phosphogluconate dh is not only an oxidation step but it’s also a decarboxylation reaction. The primary results of the pathway are: The generation of reducing equivalents, in the form of NADPH, used in reductive biosynthesis reactions within cells (e.g. fatty acid synthesis). Production of ribose-5-phosphate (R5P), used in the synthesis of nucleotides and nucleic acids. Production of erythrose-4-phosphate (E4P), used in the synthesis of aromatic amino acids. Transketolase and transaldolase reactions are similar in that they transfer between carbon chains, transketolases 2 carbon units or transaldolases 3 carbon units. Regulation; Glucose-6-phosphate dehydrogenase is the rate- controlling enzyme of this pathway. It is allosterically stimulated by NADP+. The ratio of NADPH:NADP+ is normally about 100:1 in liver cytosol. -

Biochemistry Entry of Fructose and Galactose
Paper : 04 Metabolism of carbohydrates Module : 06 Entry of Fructose and Galactose Dr. Vijaya Khader Dr. MC Varadaraj Principal Investigator Dr.S.K.Khare,Professor IIT Delhi. Paper Coordinator Dr. Ramesh Kothari,Professor UGC-CAS Department of Biosciences Saurashtra University, Rajkot-5, Gujarat-INDIA Dr. S. P. Singh, Professor Content Reviewer UGC-CAS Department of Biosciences Saurashtra University, Rajkot-5, Gujarat-INDIA Dr. Charmy Kothari, Assistant Professor Content Writer Department of Biotechnology Christ College, Affiliated to Saurashtra University, Rajkot-5, Gujarat-INDIA 1 Metabolism of Carbohydrates Biochemistry Entry of Fructose and Galactose Description of Module Subject Name Biochemistry Paper Name 04 Metabolism of Carbohydrates Module Name/Title 06 Entry of Fructose and Galactose 2 Metabolism of Carbohydrates Biochemistry Entry of Fructose and Galactose METABOLISM OF FRUCTOSE Objectives 1. To study the major pathway of fructose metabolism 2. To study specialized pathways of fructose metabolism 3. To study metabolism of galactose 4. To study disorders of galactose metabolism 3 Metabolism of Carbohydrates Biochemistry Entry of Fructose and Galactose Introduction Sucrose disaccharide contains glucose and fructose as monomers. Sucrose can be utilized as a major source of energy. Sucrose includes sugar beets, sugar cane, sorghum, maple sugar pineapple, ripe fruits and honey Corn syrup is recognized as high fructose corn syrup which gives the impression that it is very rich in fructose content but the difference between the fructose content in sucrose and high fructose corn syrup is only 5-10%. HFCS is rich in fructose because the sucrose extracted from the corn syrup is treated with the enzyme that converts some glucose in fructose which makes it more sweet. -

LACTOSE & D-GALACTOSE (Rapid)
www.megazyme.com LACTOSE & D-GALACTOSE (Rapid) ASSAY PROCEDURE K-LACGAR 02/21 Incorporating A Procedure For The Analysis Of “Low- Lactose” Or “Lactose-Free” Samples Containing High Levels Of Monosaccharides (Improved Rapid Format) (*115 Assays per Kit) * The number of tests per kit can be doubled if all volumes are halved The reagents provided in this kit are also suitable for use with AOAC method 2006.06 – Lactose in milk. Patented: US 7,785,771 B2 and EP1 828 407 (GB, FR, IE, DE) © Megazyme 2021 INTRODUCTION: Lactose, or milk sugar, is a white crystalline disaccharide. It is formed in the mammary glands of all lactating animals and is present in their milk. Lactose yields D-galactose and D-glucose on hydrolysis by lactase (β-galactosidase), an enzyme found in gastric juice. People who lack this enzyme after childhood cannot digest milk and are said to be lactose intolerant. Common symptoms of lactose intolerance include nausea, cramps, gas and diarrhoea, which begin about 30 minutes to 2 hours after eating or drinking foods containing lactose. Between 30 and 50 million Americans are lactose intolerant, with certain ethnic and racial populations being more widely affected than others; as many as 75 percent of all African-Americans and Native Americans and 90 percent of Asian-Americans are lactose intolerant. The condition is least common among persons of northern European descent. Enzymic methods for the measurement of lactose are well known and are generally based on the hydrolysis of lactose to D-galactose and D-glucose with β-galactosidase, followed by determination of either D-galactose or D-glucose. -

Glucose-Galactose Malabsorption J
Arch Dis Child: first published as 10.1136/adc.42.226.592 on 1 December 1967. Downloaded from Arch. Dis. Childh., 1967, 42, 592. Glucose-galactose Malabsorption J. M. ABRAHAM, B. LEVIN, V. G. OBERHOLZER, and ALEX RUSSELL* From Queen Elizabeth Hospitalfor Children, Hackney Road, London E.2 Primary disaccharidase deficiency is a well-known electrolytaemia. On the 16th day, therefore, feeds cause of diarrhoea in infancy. The offending were changed to Nutramigen without improvement, and disaccharide appears in the stool after ingestion due 9 days later this was substituted by Velactin. Both of to the absence in the intestinal mucosa of the appro- these low-lactose preparations contain, in addition to protein, large amounts of dextrin and starch, and priate splitting enzyme. A much rarer cause of Nutramigen also includes maltose, while Velactin also has diarrhoea in this period is a failure of absorption of glucose and sucrose. All these carbohydrates on the monosaccharides, glucose and galactose, in the digestion give rise to glucose. The diarrhoea now presence of histologically normal mucosa and full decreased and the stools became less liquid; but disaccharidase activity. The clinical and bio- there was no weight gain (Fig. 1) and glucose was still chemical picture of this newly-described entity is present in the stools. After 12 days on this regime, an characteristic. Watery diarrhoea, with glucose extensive rash developed on the buttocks and groins, and/or galactose in the stools, develops within three with stomatitis (Fig. 2), anaemia, hypokalaemia, and days of milk feeding and continues as long as the hyperchloraemic acidosis (Na 138, K 2-4, Cl 112, standard bicarbonate 13*7 mEq/l.). -

Pedi Sugar Facts
Facts About Sugar THE SUGAR BASICS READING A FOOD LABEL Sugar gives the body energy, but too much sugar is unhealthy. The body actually uses all sugars the same way- it changes them to glucose, which is what our body uses for energy. However, in order to get long-lasting energy for your body, you need to eat more complex sugars (or carbohydrates) such as whole grain products. Eating too many simple sugars, like from soda or sweets, gives you quick energy or a “sugar high” which is quickly gone, leaving you feeling sluggish. You can avoid eating too much sugar by being smart and knowing how to find it on a label. Find sugar by looking for –ose at the end. Examples: Glucose- fruits, vegetables, honey, milk, cereal Fructose- fruits, vegetables, honey Galactose- milk products Sucrose- fruits, vegetables, table sugar Find the “Total Carbohydrate”, Lactose- milk products and below it find “Sugars”. Sugar Maltose- malt products, cereal is listed in grams. Every 4 grams of sugar = 1 teaspoon of table sugar. Know the many different names for sugar, such as: corn syrup, high-fructose corn syrup, In this example, 8 oz. of lemonade dextrose, maltodextrins, granulated sugar, or concentrated fruit juice sweetener. Added (240 mL) has 27 grams of sugar. sugars can come from corn, beet, grape or This equals about 7 teaspoons, sugar cane, which are processed before being and the daily limit is 10 added to foods. Sugars can also be naturally teaspoons! occurring (in fruit) or added (in soda). May be reproduced for educational purposes ©2007 Developed by graduate nutrition students at Framingham State College Facts About Sugar HIGH FRUCTOSE CORN SYRUP DID YOU KNOW??? High Fructose Corn Syrup (HFCS) is an One 12-oz. -

Long-Term Storage of Sugar Beet in North-West Europe
Coordination Beet Research COBRI International Report No. 1 – 2013 Long-term storage of sugar beet in North-West Europe ơ% Long-term storage of sugar beet in North-West Europe Toon Huijbregts (IRS), Guy Legrand (IRBAB / KBIVB), ơȋ ȌǡȋȌǡ%ȋȌ Ǥ͙͘Ȃ͚͙͛͘ CONTENTS PREFACE. 5 SUMMARY . 7 1. INTRODUCTION . 8 2. BACKGROUND TO QUALITY REDUCTION DURING STORAGE . 10 2.1 Quality Assessment . 10 2.2 Respiration . 10 2.3 Wound Healing . 11 2.4 Sprouts . 11 2.5 Root Rot . 11 2.6 Bacteria. 12 2.7 Moulds . 12 2.8 Frost Damage . 13 2.9 Dirt Tare. .14 3. ANALYTICAL METHODS. 15 3.1 Storage experiments . 15 3.1.1 Respiration measurements . 15 3.1.2 Storage under controlled conditions . 16 3.1.3 Clamp experiments . 17 3.2 Analyses . 18 3.2.1 Standard quality parameters: sugar (Pol), potassium, sodium, amino nitrogen . 18 $GGLWLRQDOTXDOLW\SDUDPHWHUVVXFURVHLQYHUWVXJDUVUDI¿QRVHVROXEOHQLWURJHQ . 18 3.2.3 Scores for sprouts, frozen parts, moulds, rot. 20 'HWHFWLRQRIUHYHUVLEOHDQGLUUHYHUVLEOHIURVWGDPDJH . 21 4. FACTORS AFFECTING STORABILITY . 21 4.1 Beet material. 21 4.1.1 Beet growing conditions . 21 4.1.2 Variety . 22 4.1.3 Beet size . 24 4.2 Harvesting and clamping conditions . 24 4.2.1 Root damage during harvesting and clamping . 24 4.2.2 Defoliation and topping . 27 4.2.3 Weather conditions during harvesting and storage . 29 4.3 Storage conditions. 29 4.3.1 Storage temperature and period . 29 4.3.2 Humidity. 32 4.3.3 Frost damage . 32 4.3.4 Treatments. 34 5. OPTIMAL HARVEST AND CLAMP MANAGEMENT . 35 5.1 Aim. -

Galactose, Natural Sources and Biotech Uses
Galactose, Natural Sources and Biotech Uses Nowadays, as the science developed, people are more and more interested in two hot topics, global environment and human health. In the near future, as scientists predict, it’s very likely for human to have a new “clean” biofuel coming from carbohydrates and replacing the fossil fuel we are using now, which would be a huge advancement of environmental protection. Also people are learning more about the minute quantity but essential contents in diet, like so-called bifidus factors, which have big affects on health, especially for babies. And interestingly, all of these can be related to the “half milk sugar” (from Chinese) galactose. In this essay, I will give a broad overview of galactose, including its nature source, some functions, related enzymes and future prospects. As from many aspects, galactose would have an important role in future and more sources would be needed, both natural and artificial. So where to start finding new sources of galactose would be an interesting question. And in this essay, I will try to give some opinions to answer this question. 1. What is galactose? Galactose, also named D-galactopyranose, is a simple monosaccharide sugar, sharing the same molecular chemical formula C6H12O6 with glucose and fructose, while they are defined as structure isomers as they have different structures. Galactose has the appearance of white crystal and is less sweet comparing to glucose or fructose. Galactose has an average mass of 180.16 Da. As galactose is consisting of six carbon and one aldehyde group, it is classified as an aldohexose. -
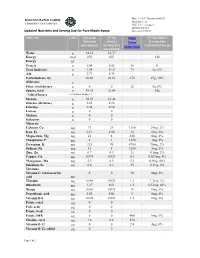
Updated Nutrients and Serving Size for Pure Maple Syrup: Daily Value
Date: 1/15/17 Document #1017 BASCOM MAPLE FARMS Revision #: A COMPANY DOCUMENT SQF 2.3.4 (2 pages) Revised 9/25/18 Updated Nutrients and Serving Size for Pure Maple Syrup: Reviewed 8/26/19 Nutrient Units 100 gram 39.78g Daily 39.78g (30mL) Nutrients (30mL) Value Serving Size (unrounded) Serving Size (DRV/RDI) (Nutritional Facts) (unrounded) Water g 32.15 12.79 Energy kcal 270 107 110 Energy kJ Protein g 0.04 0.02 50 0 Total lipid (fat) g 0.24 0.10 78 0g, 0% Ash g 0.71 0.28 Carbohydrate, by 66.89 26.61 275 27g, 10% difference g Fiber, total dietary g 0 0 28 0g, 0% Sugars, total g 59.92 23.84 24g *Added Sugars see bottom of page 2 Sucrose g 58.93 23.44 Glucose (dextrose) g 0.65 0.26 Fructose g 0.34 0.14 Lactose g 0 0 Maltose g 0 0 Galactose g 0 0 Minerals Calcium, Ca mg 73 29 1300 29mg, 2% Iron, Fe mg 0.11 0.04 18 0mg, 0% Magnesium, Mg mg 21 8 420 8mg, 2% Phosphorus, P mg 2 1 1250 1mg, 0% Potassium, K mg 225 90 4700 90mg, 2% Sodium, Na mg 12 5 2300 5mg, 0% Zinc, Zn mg 0.7 0.3 11 0.3mg, 2% Copper, Cu mg 0.074 0.029 0.9 0.029mg, 4% Manganese, Mn mg 2.3 0.9 2.3 0.9mg, 40% Selenium, Se µg 0.6 0.2 55 0.2mg, 0% Vitamins Vitamin C, total ascorbic 0 0 90 0mg, 0% acid mg Thiamin mg 0.066 0.026 1.2 1.2mg, 2% Riboflavin mg 1.27 0.51 1.3 0.51mg, 40% Niacin mg 0.081 0.032 16 0mg, 0% Pantothenic acid mg 0.03 0.01 5 0mg, 0% Vitamin B-6 mg 0.002 0.001 1.7 0mg, 0% Folate, total µg 0 0 Folic acid µg 0 0 Folate, food µg 0 0 Folate, DFE µg 0 0 400 0mg, 0% Choline, total mg 1.6 0.6 550 Vitamin B-12 µg 0 0 2.4 0µg, 0% Vitamin B-12, added µg 0 0 Page 1 of 2 Vitamin -

Carbohydrates: Simple Sugars and Complex Chains
Chapter 4 Carbohydrates: Simple Sugars and Complex Chains THINK About It 1 When you think of the word carbohydrate , what foods come to mind? 2 Fiber is an important part of a healthy diet—are you eating enough? 3 Is honey more nutritious than white sugar? What do you think? 4 What are the downsides to including too many carbohydrates in your diet? LEARNING Objectives 1 Di erentiate among disaccharides, oligosaccharides, and polysaccharides. 2 Explain how a carbohydrate is digested and absorbed in the body. 3 Explain the functions of carbohydrates in the body. 4 Make healthy carbohydrate selections for an optimal diet. 5 Analyze the contributions of carbohydrates to health. © Seregam/Shutterstock, Inc. Seregam/Shutterstock, © 9781284086379_CH04_095_124.indd 95 26/02/15 6:13 pm 96 CHAPTER 4 CARBOHYDRATES: SIMPLE SUGARS AND COMPLEX CHAINS oes sugar cause diabetes? Will too much sugar make a child hyper- Quick Bite active? Does excess sugar contribute to criminal behavior? What Is Pasta a Chinese Food? D about starch? Does it really make you fat? These and other ques- Noodles were used in China as early as the fi rst tions have been raised about sugar and starch—dietary carbohydrates—over century; Marco Polo did not bring them to Italy the years. But, where do these ideas come from? What is myth, and what until the 1300s. is fact? Are carbohydrates important in the diet? Or, as some popular diets suggest, should we eat only small amounts of carbohydrates? What links, if any, are there between carbohydrates in your diet and health? Most of the world’s people depend on carbohydrate-rich plant foods for daily sustenance. -

Facts About Sugars – Brochure
1. INTRODUCTION Sugars are found in nature. All green plants providing foodstuffs, including fruits and vegetables, grains, as well as milk and honey, contain naturally-occurring sugars. Many types of sugars are found in the diet on a daily basis. These include, for example, glucose, fructose, sucrose, and lactose. When the term ‘sugar’ is used, people are referring to ‘sucrose’ (table sugara). Over recent years, a debate has arisen over the amount of sugars people should eat and the potential effects sugars may have on health. This brochure explores the science around sugars and intends to put the evidence on sugars and health into context. a Table sugar is produced from sugar beet or sugar cane. 1 1.1 Biochemistry of sugars Sugars are carbohydratesb. As such, they provide the body with the energy that our organs and muscles need to function. Sugars are typically named in relation to their size and chemical structure (Figure 1). Monosaccharides are single unit sugars with ‘mono’ meaning ‘one’ and ‘saccharide’ meaning sugar mol- ecule. Monosaccharides commonly found in food are glucose, fructose and galactose. Disaccharides consist of two monosaccharides joined together. Disaccharides commonly found in food are sucrose (glucose + fructose), lactose (glucose + galactose) and maltose (glucose + glucose). Oligo - and polysaccharides contain more than two monosaccharides joined together. Starch is a well- known polysaccharide. Other examples include fructo-oligosaccharides and malto-oligosaccharides. Oligo - and polysaccharides are sometimes called ‘complex carbohydrates’ in recognition of their size dif- ference from mono- and disaccharides, which are classed as ‘simple sugars’ due to their small size. It is mono- and disaccharides which are the focus of this brochure. -

Diagnosing and Treating Intolerance to Carbohydrates in Children
nutrients Review Diagnosing and Treating Intolerance to Carbohydrates in Children Roberto Berni Canani 1,2,3,*, Vincenza Pezzella 1, Antonio Amoroso 1, Tommaso Cozzolino 1, Carmen Di Scala 1 and Annalisa Passariello 1 1 Department of Translational Medical Science, University of Naples “Federico II”, 80131 Naples, Italy; [email protected] (V.P.); [email protected] (A.A.); [email protected] (T.C.); [email protected] (C.D.S.); [email protected] (A.P.) 2 European Laboratory for the Investigation of Food Induced Diseases, University of Naples “Federico II”, 80131 Naples, Italy 3 CEINGE Advanced Biotechnologies, University of Naples “Federico II”, 80131 Naples, Italy * Correspondence: [email protected]; Tel.: +39-081-746-2680 Received: 27 October 2015; Accepted: 25 February 2016; Published: 10 March 2016 Abstract: Intolerance to carbohydrates is relatively common in childhood, but still poorly recognized and managed. Over recent years it has come to the forefront because of progresses in our knowledge on the mechanisms and treatment of these conditions. Children with intolerance to carbohydrates often present with unexplained signs and symptoms. Here, we examine the most up-to-date research on these intolerances, discuss controversies relating to the diagnostic approach, including the role of molecular analysis, and provide new insights into modern management in the pediatric age, including the most recent evidence for correct dietary treatment. Keywords: lactose intolerance; fructose malabsorption; sucrase-isomaltase deficiency; glucose-galactose malabsorption; sorbitol intolerance; trehalose intolerance; FODMAPs intolerance; breath test; molecular analysis 1. Introduction Adverse food reactions (AFR) represent a relevant problem in daily clinical practice, but are poorly recognized and managed. -

Sugar Beet Pulp
Ethanol Production from Sugar Beet Pulp Sugar Beet Pulp To determine the technical feasibility of sequential enzyme treatments to produce separate fermentable Pectinases & sugar streams to increase cellulose loading Hemicellulases rates and ethanol titers from sugar fermentation. Separate sugar streams also may be fermented into Enzymatic Cellulose other higher-value fuels or chemicals. Objective Hydrolysis Cellulases Arabinose and • Minimal additional costs for harvest, storage or Galacturonic Acid Glucose transportation of sugar beet pulp Sugar beet plant • Elimination of drying and pressing processes for pulp; energy for drying is estimated at $80/ton Escherichia coli Saccharomyces • Low lignin content (<2%) leads to elimination of Fermentation cerevisiae costly thermochemical pretreatment required for Process most other biomass feedstocks Benefits • Sugar beet pulp has a high carbohydrate content Ethanol consisting mainly of cellulose, hemicellulose and pectin Flow chart of ethanol production procedure from sugar beet pulp • Cellulose, hemicellulose and pectin components are readily hydrolyzed by commercial enzymes • Relatively small production scale on a processing plant basis • Relatively low level of cellulose content • Large pectin levels not typical of most lignocellulosic biomass Challenges • Hemicellulose component has different composition than most biomass feedstocks Working with sugar Bioproduct Research Analyzing carbohydrates beet pulp Laboratory and ethanol Cellulose • North Dakota and Minnesota produce 15.8 Other million