Dialects in Animals: Evidence, Development and Potential Functions
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

The Relationships of the Starlings (Sturnidae: Sturnini) and the Mockingbirds (Sturnidae: Mimini)
THE RELATIONSHIPS OF THE STARLINGS (STURNIDAE: STURNINI) AND THE MOCKINGBIRDS (STURNIDAE: MIMINI) CHARLESG. SIBLEYAND JON E. AHLQUIST Departmentof Biologyand PeabodyMuseum of Natural History,Yale University, New Haven, Connecticut 06511 USA ABSTRACT.--OldWorld starlingshave been thought to be related to crowsand their allies, to weaverbirds, or to New World troupials. New World mockingbirdsand thrashershave usually been placed near the thrushesand/or wrens. DNA-DNA hybridization data indi- cated that starlingsand mockingbirdsare more closelyrelated to each other than either is to any other living taxon. Some avian systematistsdoubted this conclusion.Therefore, a more extensiveDNA hybridizationstudy was conducted,and a successfulsearch was made for other evidence of the relationshipbetween starlingsand mockingbirds.The resultssup- port our original conclusionthat the two groupsdiverged from a commonancestor in the late Oligoceneor early Miocene, about 23-28 million yearsago, and that their relationship may be expressedin our passerineclassification, based on DNA comparisons,by placing them as sistertribes in the Family Sturnidae,Superfamily Turdoidea, Parvorder Muscicapae, Suborder Passeres.Their next nearest relatives are the members of the Turdidae, including the typical thrushes,erithacine chats,and muscicapineflycatchers. Received 15 March 1983, acceptedI November1983. STARLINGS are confined to the Old World, dine thrushesinclude Turdus,Catharus, Hylocich- mockingbirdsand thrashersto the New World. la, Zootheraand Myadestes.d) Cinclusis -

Hand-Raising and Rehabilitation of Mynas
Hand-raising and rehabilitation of mynas Corina Gardner Hill mynas David Lim Introduction Mynas are average sized (about 22-28 cms) passerine birds which belong to the family of starlings, Sturnidea. The term ‘myna’ is commonly used to refer to starlings in India. Mynas are commonly distributed throughout Southern and Eastern Asia. These birds have duller plumage and are more terrestrial compared to other members of the starling family. 1 Rehabber’s Den © 2012 Hand-raising and rehabilitation of mynas Common myna Acridotheres tristis The common myna is widely distributed throughout India and Asia and has also been introduced to many parts of the world. The species lives in woodlands and near human habitations. They have brown plumage, a black head, throat and breast, while the bill and legs are yellow. They also have a distinctive yellow patch behind the eyes. They are omnivorous birds and will scavenge for just about anything including discarded scraps, insects, seeds, grain and fruit. They roost in large trees and build their nests in walls and rooftops of buildings. Common myna Tris Jungle myna Acridotheres fuscus Jungle mynas are found in and around the Indian subcontinent. They have brownish grey plumage, a tuft of feathers on their heads, white patches on their 2 Rehabber’s Den © 2012 Hand-raising and rehabilitation of mynas primaries and a white tipped tail. They typically live in forests, tea plantations and near paddy fields. They are omnivorous birds and their diet often consists of insects, fruit, seeds and even nectar from flowers. Jungle myna Devna Arora Bank myna Acridotheres ginginianus This species of myna is found primarily in the Indian subcontinent. -

Sumatran Tiger - One of the Most Incredible and Unlikely Sightings Ever on a Birdquest Tour! (Pete Morris)
THE Sumatran Tiger - one of the most incredible and unlikely sightings ever on a Birdquest tour! (Pete Morris) SUMATRA 6 – 21/26/30 JUNE 2014 LEADER: PETE MORRIS It’s not often that I begin a birding tour report with a mammal, but our incredible sighting of Sumatran Tiger I’m afraid stole the show from the birds this time around! It’s rarely seen let alone photographed at point blank range and watched for 20 minutes, so we can certainly class ourselves as part of a very select club! Fortu- nately the birds did us proud too! It had been a few years since I’d led this tour, and I’d almost forgotten how challenging the birding can be! Fortunately, I hadn’t forgotten that if you work hard and keep plugging away, success ultimately comes your way, and I was lucky to have a team of stalwarts that were prepared to, at times, put in the hard yards to gain the rewards! And in the end, we were extremely successful in tracking down nearly 1 BirdQuest Tour Report: Sumatra www.birdquest-tours.com Nightbirds were a theme of the tour, and Reddish Scops Owl an endearing example! (Pete Morris) all of our hoped for targets. The main tour focused on three areas. At the imposing Gunung Kerinci we tracked down a great selection of specialities, including Red-billed Partridge, the rare Javan Woodcock (in daylight), Sumatran Trogon, a confiding Schneider’s Pitta, fabulous Sumatran and Rusty-breasted Wren-Babblers, Sun- da Forktail (nearby), Brown-winged and Shiny Whistling Thrushes and even a brief Sumatran Cochoa. -
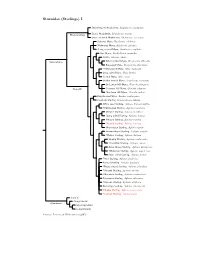
Sturnidae Tree, Part 1
Sturnidae (Starlings) I Stripe-headed Rhabdornis, Rhabdornis mystacalis Grand Rhabdornis, Rhabdornis grandis Rhabdornithini Stripe-breasted Rhabdornis, Rhabdornis inornatus Sulawesi Myna, Basilornis celebensis ?Helmeted Myna, Basilornis galeatus ?Long-crested Myna, Basilornis corythaix Apo Myna, Goodfellowia mirandus Coleto, Sarcops calvus Graculinae White-necked Myna, Streptocitta albicollis Bare-eyed Myna, Streptocitta albertinae ?Yellow-faced Myna, Mino dumontii Long-tailed Myna, Mino kreffti Golden Myna, Mino anais Golden-crested Myna, Ampeliceps coronatus Sri Lankan Hill-Myna, Gracula ptilogenys Graculini Common Hill Myna, Gracula religiosa ?Southern Hill Myna, Gracula indica Fiery-browed Myna, Enodes erythrophris Grosbeak Starling, Scissirostrum dubium White-eyed Starling, Aplonis brunneicapillus ?Yellow-eyed Starling, Aplonis mystacea Metallic Starling, Aplonis metallica ?Long-tailed Starling, Aplonis magna Pohnpei Starling, Aplonis pelzelni ?Kosrae Starling, Aplonis corvina Micronesian Starling, Aplonis opaca Brown-winged Starling, Aplonis grandis ?Makira Starling, Aplonis dichroa Singing Starling, Aplonis cantoroides ?Tanimbar Starling, Aplonis crassa Asian Glossy Starling, Aplonis panayensis ?Moluccan Starling, Aplonis mysolensis Short-tailed Starling, Aplonis minor ?Atoll Starling, Aplonis feadensis Rennell Starling, Aplonis insularis ?Rusty-winged Starling, Aplonis zelandica ?Striated Starling, Aplonis striata ?Mountain Starling, Aplonis santovestris Polynesian Starling, Aplonis tabuensis ?Samoan Starling, Aplonis atrifusca Rarotonga Starling, Aplonis cinerascens ?Mauke Starling, Aplonis mavornata ?Tasman Starling, Aplonis fusca Sturnini Cinnyricinclini Sturninae Onychognathini Lamprotornini Sources: Lovette and Rubenstein (2007).. -

A Resume of Anting, with Particular Reference to A
A RESUMI? OF ANTING, WITH PARTICULAR REFERENCE TO A CAPTIVE ORCHARD ORIOLE BY LOVIE M. WHITAKER INCE Audubon (1831:7) wrote of Wild Turkeys (Meleugris gallopuvo) S rolling in “deserted” ants ’ nests (Allen, 1946)) and Gosse (1847:225) reported Tinkling Grackles (Q uiscalus niger) in nature anointing themselves with lime fruits (Chisholm, 1944), an extensive literature on the anting ac- tivities of birds has slowly evolved. The complete bibliography of anting probably would approximate 250 items, yet the purpose of the behavior re- mains unexplained. Anting may be defined as the application of foreign substances to the plum- age and possibly to the skin. These substances may be applied with the bill, or the bird may “bathe” or posture among thronging ants which invest its plumage. Among numerous explanations for the use of ants are these: (1) the bird wipes off ant acid, preparatory to eating the ant; (2) ants prey upon, and their acids repel, ectoparasites; (3) ant acids have tonic or medicinal effects on the skin of birds; (4) odor of ants attracts birds, much as dogs are drawn to ordure or cats to catnip; (5) an t s intoxicate the bird or give it unique pleasurable effects; (6) ant substances on the plumage, irradiated by sun- light, produce vitamin D, which the bird ingests during preening; (7) the bird enjoys the movement of insects in its plumage; (8) ant substances pre- vent over-drying of feather oils or give a proper surface film condition to the feathers. For discussions of these possibilities, see Chisholm (1944, 1948: 163-175)) Adlersparre (1936)) IJ zen d oorn (1952~)) Eichler (1936~)) Klein- Schmidt (in Stresemann, 1935b), L ane (1951:163-177)) Kelso (1946, 1949, 1950a, 19506, 1955 :37-39)) Brackbill (1948)) G6roudet (1948), Groskin (1950)) and McAtee (1938). -

Progression of the Treasure Island Ranger Programme Bird Focused Monitoring Transects
EAZA Treasure Island Update Progression of the Treasure Island Ranger Programme Bird Focused Monitoring Transects 19/10/2020 1. Bird Monitoring Methodology Development and Implementation 1.1. Methodology Development Over the last three months, EcosystemImpact has been working with members of EAZA Silent Forest Campaign, International Union for the Conservation of Nature (IUCN) Species Survival Commission (SSC) Asian Songbird Trade Specialist Group (ASTSG) and University of Singapore Avian Evolution Lab to the development and implementation of the Treasure Island bird monitoring, anti-poaching and transect methodology. Playing a particularly important role in the development of this methodology have been EAZA’s Simon Bruslund and Tomas Ouhel; David Jeggo and Jessica Lee (also a member of Wildlife Reserves Singapore) from ASTSG; and members of Avian Evolution Lab, especially Frank Erwin Rheindt. Each name mentioned above is a regionally influential and internationally recognised bird conservationist. Tomas Ouhel, Jessica Lee and Frank Erwin Rheindt have contributed to multiple scientific journal articles on Asian bird species and the Asian Songbird Crisis, some of which are referenced below. EcosystemImpact feels privileged to sit within this network of conservationists, and to have had their assistance in the development of the Treasure Island Ranger Programme. The methodology has been developed with a focus on birds, primarily Nias hill myna(Gracula robusta), silvery pigeon (Columba argentina), along with ASTSG Tier 1 (highest priority) species, white-rumped (Barusan) shama (Copsychus [malabaricus] melanurus) and a number of other ASTSG and IUCN target species (see Table 1. below for a list of all programme target species). These species have been chosen as the programmes focus species by the people listed in the paragraph above, as they are species considered locally, regionally and internationally important and under threat. -

Bangkaru Island: the Challenges and Successes of Protecting One of Indonesia’S Last Refuges for Three Critically Endangered Taxa
22 BirdingASIA 35 (2021): 22–26 OBC!FUNDED CONSERVATION Bangkaru Island: the challenges and successes of protecting one of Indonesia’s last refuges for three Critically Endangered taxa TOM AMEY Introduction Bangkaru Island, the largest uninhabited island in (1997–2013), then Forest, Nature and Environment the Pulau Banyak archipelago, lies 75 km west of of Aceh (2015–2019), and most recently Ecosystem Sumatra and is part of Aceh province (Figure 1). Impact Foundation (EcosystemImpact) from 2019 The neighbouring islands of Tuangku and Balai host to the present day. As a result, the island’s three small communities. The Pulau Banyak biodiversity has remained intact to a degree that archipelago is part of the west Sumatran (or is almost unrivalled in the region. Barusan) island chain which extends along the west coast of Sumatra. Many of the Barusan Islands have Species of interest either never been connected to Sumatra, or have not Bangkaru is home to Nias Hill Myna Gracula been connected since the Quaternary Period, which [religiosa] robusta; Silvery Pigeon Columba has led to high levels of endemism within the chain. argentina; an undescribed/unidentified shama, Bangkaru lies within the Kepulauan Banyak possibly an endemic subspecies of Barusan Shama Taman Wisata Alam (TWA, or Nature Tourism Copsychus [malabaricus] melanurus; a subspecies Park), which confers landscape-level conservation of Brown Wood-owl Strix leptogrammica protection and is managed by Balai Konservasi nyctiphasma endemic to the Banyak Islands; a Sumber Daya Alam (BKSDA), Indonesia’s subspecies of Green Imperial-pigeon Ducula aenea government sector for natural resources and consobrina endemic to the Barusan Islands; and Black-naped Oriole Oriolus chinensis mundus and Having never been subjected to development Asian Glossy Starling Aplonis panayensis altirostris. -

Proposals 2018-C
AOS Classification Committee – North and Middle America Proposal Set 2018-C 1 March 2018 No. Page Title 01 02 Adopt (a) a revised linear sequence and (b) a subfamily classification for the Accipitridae 02 10 Split Yellow Warbler (Setophaga petechia) into two species 03 25 Revise the classification and linear sequence of the Tyrannoidea (with amendment) 04 39 Split Cory's Shearwater (Calonectris diomedea) into two species 05 42 Split Puffinus boydi from Audubon’s Shearwater P. lherminieri 06 48 (a) Split extralimital Gracula indica from Hill Myna G. religiosa and (b) move G. religiosa from the main list to Appendix 1 07 51 Split Melozone occipitalis from White-eared Ground-Sparrow M. leucotis 08 61 Split White-collared Seedeater (Sporophila torqueola) into two species (with amendment) 09 72 Lump Taiga Bean-Goose Anser fabalis and Tundra Bean-Goose A. serrirostris 10 78 Recognize Mexican Duck Anas diazi as a species 11 87 Transfer Loxigilla portoricensis and L. violacea to Melopyrrha 12 90 Split Gray Nightjar Caprimulgus indicus into three species, recognizing (a) C. jotaka and (b) C. phalaena 13 93 Split Barn Owl (Tyto alba) into three species 14 99 Split LeConte’s Thrasher (Toxostoma lecontei) into two species 15 105 Revise generic assignments of New World “grassland” sparrows 1 2018-C-1 N&MA Classification Committee pp. 87-105 Adopt (a) a revised linear sequence and (b) a subfamily classification for the Accipitridae Background: Our current linear sequence of the Accipitridae, which places all the kites at the beginning, followed by the harpy and sea eagles, accipiters and harriers, buteonines, and finally the booted eagles, follows the revised Peters classification of the group (Stresemann and Amadon 1979). -

The Common Myna, Atiu, Cook Islands J
The distribution and abundance of the common myna, Atiu, Cook Islands J. Mitchell, 2009 The distribution and abundance of an invasive species: the common myna (Acridotheres tristis) on Atiu, Cook Islands. Photos by J. Mitchell, and from Google Earth Jessica Mitchell ID: 200225474 MSc Biodiversity and Conservation Project 2009 - 1 - The distribution and abundance of the common myna, Atiu, Cook Islands J. Mitchell, 2009 Contents Figure 1. Location of the Cook Islands, South Pacific Ocean……………….…………......…4 Abstract …………………………………………………………………………….…….........5 1. Introduction ……………………………………………………………………..…………6 1.1. Invasive species………………………………...………………..………………….6 1.2. Islands Ecosystems………………………………………………………………….8 2. Study Area ………………………………………………………………………………….8 2.1. The Cook Islands……………………………………………………………………8 2.2. Atiu…………………………………………………………………………………10 Figure 2. Satellite Image of Atiu ………………………………………………...10 2.3 Vegetation and Avifauna…………………………………………………………...10 2.4. Rimatara Lorikeet………………………………………………………………….11 2.5. Invasive Species Management……………………………………………………..12 3. Study Species .......................................................................................................................13 3.1. The Common Myna……………………………………………………………....13 3.2. Eradication………………………………………………………………………..15 4. Aim ………………………………………………………………………………………...17 5. Method …………………………………………………………………………………….17 5.1. Roost Counts……………………………………………………………………..17 5.2. Transects……………………………………………………........……………....18 Figure 3. Map of Roosts and Roost Count Locations………………………...20 -

The Asian Songbird Crisis
The Asian songbird crisis: just how bad is it, and what can we do? Nigel Collar INDONESIA Jepson 2005–2011 Jepson 2005–2011 Jepson 2005–2011 Jepson 2005–2011 Jepson 2005–2011 Shepherd TRAFFIC 2004–2017 Shepherd TRAFFIC 2004–2017 Shepherd TRAFFIC 2004–2017 Shepherd TRAFFIC 2004–2017 Shepherd TRAFFIC 2004–2017 Shepherd TRAFFIC 2004–2017 Shepherd TRAFFIC 2004–2017 Shepherd TRAFFIC 2004–2017 Nijman 2010–2017 Nijman 2010–2017 TASA/associates 2012–2017 TASA/associates 2012–2017 TASA/associates 2012–2017 TASA/associates 2012–2017 TASA/associates 2012–2017 TASA/associates 2012–2017 Harris 2015–2017 Harris 2015–2017 BirdLife Magazine Asian Geographic Five species when TASA formed in 2011 IUCN Red List for Indonesian songbirds 2017 Javan Green Magpie Cissa thalassina CR Straw-headed Bulbul Pycnonotus zeylanicus EN Ruby-throated Bulbul Pycnonotus dispar VU Aceh Bulbul Pycnonotus snouckaerti VU Javan White-eye Zosterops flavus VU Rufous-fronted Laughingthrush Garrulax rufifrons CR Sumatran Laughingthrush Garrulax bicolor EN Sumatran Mesia Leiothrix laurinae EN Bali Myna Leucopsar rothschildi CR Black-winged Myna Acridotheres melanopterus CR Grey-backed Myna Acridotheres tricolor CR Grey-rumped Myna Acridotheres tertius CR Javan Myna Acridotheres javanicus VU Asian Pied Starling Gracupica jalla CR Tenggara Hill Myna Gracula venerata EN Nias Hill Myna Gracula robusta CR Sumatran Leafbird Chloropsis media VU Greater Green Leafbird Chloropsis sonnerati VU Java Sparrow Lonchura oryzivora VU White-rumped Shama LC, but with CR island subspecies tricolor -

Catalogue of Type Specimens 4. Linnaean Specimens
Uppsala University Museum of Evolution Zoology section Catalogue of type specimens. 4. Linnaean specimens 1 UPPSALA UNIVERSITY, MUSEUM OF EVOLUTION, ZOOLOGY SECTION (UUZM) Catalogue of type specimens. 4. Linnaean specimens The UUZM catalogue of type specimens is issued in four parts: 1. C.P.Thunberg (1743-1828), Insecta 2. General zoology 3. Entomology 4. Linnaean specimens (this part) Unlike the other parts of the type catalogue this list of the Linnaean specimens is heterogenous in not being confined to a physical unit of material and in not displaying altogether specimens qualifying as types. Two kinds of links connect the specimens in the list: one is a documented curatorial tradition referring listed material to collections handled and described by Carl von Linné, the other is associated with the published references by Linné to literary or material sources for which specimens are available in the Uppsala University Zoological Museum. The establishment of material being 'Linnaean' or not (for the ultimate purpose of a typification) involves a study of the history of the collections and a scrutiny of individual specimens. An important obstacle to an unequivocal interpretation is, in many cases, the fact that Linné did not label any of the specimens included in the present 'Linnaean collection' in Uppsala (at least there are no surviving labels or inscriptions with his handwriting or referable to his own marking of specimens; a single exception will be pointed out below in the historical survey). A critical examination must thus be based on the writings of Linné, a consideration of the relation between between these writings and the material at hand, and finally a technical and archival scrutiny of the curatorial arrangements that have been made since Linné's time. -

News Update Prigenark 27.03.2020
Updates from Prigen Conservation Breeding Ark. 27.03.2020 Jochen Menner All of you who have contributed to the development of Prigen Conservation Breeding Ark have made an investment into the creation of a brighter future. In return, we want to brighten these dark days with a few joyful updates from Prigen. Our first rufous-fronted Laughingthrush chick To begin with, we would like to inform you that we are all healthy and we continue to run PCBA as usual. Since our host, Taman Safari was forced to close its gates to visitors which resulted in the temporary reduction of staff numbers, we faced some uncertainties. Luckily, those were managed with great understanding by TSI, so that we are able to keep operating in a reasonable manner. Additionally, over the last couple of days we experienced great solidarity from Vogelpark Marlow, ZGAP, and Roland Wirth who have made spontaneous donations to cover the shortages we faced which allowed us to keep working as usual and in the best interest of our animals! While around us the world comes more and more to a halt, we are still seeing great progress on our new projects and in breeding. Unit 4 Our Unit 4 had been completed in January 2020. With 11 aviaries of 7m×2.5m×5m, we now have the opportunity to house our most sensitive pairs in a way that allows the birds to get away from the keepers when they need to enter the aviaries for the regular feeding and cleaning duties. In this arrangement, the birds feel safer than in smaller aviaries.