Androgen Insensitivity Syndrome
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Hypospadias by Ronald S
Kapi‘olani Pediatric Urology Hypospadias By Ronald S. Sutherland, M.D., F.A.A.P., F.A.C.S. What is hypospadias? Defined as a congenital deformity where the urethral opening is located beneath the penis rather then the tip, hypospadias ranges from a mild to severe deformity. The more common form is the distal variety, with the opening toward the front of the penis, which can usually be repaired with pleasing cosmetic results. More severe forms may be associated with the opening located at the base of the penis or further back near the anus. Usually hypospadias is also present with downward curvature of the penis (chordee), and a flattening of the foreskin with a hood-like covering. Occasionally the scrotum is also malformed and appears higher around the penis. Hypospadias can be found in a variety of congenital syndromes, including those with cardiac, renal, and testicular anomalies. (see next page) What causes hypospadias? Hypospadias develops early in gestation and occurs for unknown reasons, although there is slight familial tendency. Recent evidence suggests that the incidence is increasing and may be linked to environmental and genetic disruptions during the period when genital development is particularly sensitive to sex-steroid hormone imbalances. Occasionally, hypospadias can be detected by prenatal ultrasound. No pre-natal treatment or intervention is available. Parents should not request a circumcision for their newborn son with hypospadias because the foreskin may be necessary to assist with surgical repair. Sometimes hypospadias is not recognized until after the circumcision is completed. These cases are usually mild forms that can be repaired without the use of foreskin. -

Next Generation Sequencing Panels for Disorders of Sex Development
Next Generation Sequencing Panels for Disorders of Sex Development Disorders of Sex Development – Overview Disorders of sex development (DSDs) occur when sex development does not follow the course of typical male or female patterning. Types of DSDs include congenital development of ambiguous genitalia, disjunction between the internal and external sex anatomy, incomplete development of the sex anatomy, and abnormalities of the development of gonads (such as ovotestes or streak ovaries) (1). Sex chromosome anomalies including Turner syndrome and Klinefelter syndrome as well as sex chromosome mosaicism are also considered to be DSDs. DSDs can be caused by a wide range of genetic abnormalities (2). Determining the etiology of a patient’s DSD can assist in deciding gender assignment, provide recurrence risk information for future pregnancies, and can identify potential health problems such as adrenal crisis or gonadoblastoma (1, 3). Sex chromosome aneuploidy and copy number variation are common genetic causes of DSDs. For this reason, chromosome analysis and/or microarray analysis typically should be the first genetic analysis in the case of a patient with ambiguous genitalia or other suspected disorder of sex development. Identifying whether a patient has a 46,XY or 46,XX karyotype can also be helpful in determining appropriate additional genetic testing. Abnormal/Ambiguous Genitalia Panel Our Abnormal/Ambiguous Genitalia Panel includes mutation analysis of 72 genes associated with both syndromic and non-syndromic DSDs. This comprehensive panel evaluates a broad range of genetic causes of ambiguous or abnormal genitalia, including conditions in which abnormal genitalia are the primary physical finding as well as syndromic conditions that involve abnormal genitalia in addition to other congenital anomalies. -

Hypospadias Repair
Patient and Family Education Hypospadias Repair What causes it? What is hypospadias? Incomplete development of the urethra causes Hypospadias is a birth defect of the penis. hypospadias. It can occur in families. The The urethral opening, the hole where the urine comes out, is not in the normal position. reason for it happening is usually not known. Instead of the tip, it is on the undersurface of Why is it important to recognize the penis. Boys with hypospadias are usually hypospadias? missing the underside of their foreskin so An abnormally placed urethral opening does that the foreskin forms a hood. For this not allow the urine to pass as it s hould. A reason, most boys born with boy with hypospadias urinates with a hypospadias are not circumcised. There is stream that is often directed downward often a bend or curve (called chordee) in the rather than out and away from the body. This penis when the boy has an erection. causes wet pants and shoes. This condition, Hypospadias may be mild, moderate, or when left uncorrected, may make future severe depending on how far back the opening sexual intercourse difficult, or is and how much curvature is present. The impossible. more severe forms of hypospadias are usually associated with worsening degrees of chordee. Can it be corrected? Yes, surgery can correct the problem. These operations are best done between 6 and 18 months of age. The repair is usually performed in one surgery. If the hypospadias is severe, it may be necessary to have more than one surgery. The Normal position for urethra surgery usually lasts 1-3 hours and the patient goes home the same day. -

Association of the Rectovestibular Fistula with MRKH Syndrome And
Association of the rectovestibular fistula with MRKH Syndrome and the paradigm Review Article shift in the management in view of the future uterine transplant © 2020, Sarin YK Yogesh Kumar Sarin Submitted: 15-06-2020 Accepted: 30-09-2020 Director Professor & Head Department of Pediatric Surgery, Lady Hardinge Medical College, New Delhi, INDIA License: This work is licensed under Correspondence*: Dr. Yogesh Kumar Sarin, Director Professor & Head Department of Pediatric Surgery, Lady a Creative Commons Attribution 4.0 Hardinge Medical College, New Delhi, India, E-mail: [email protected] International License. DOI: https://doi.org/10.47338/jns.v9.551 KEYWORDS ABSTRACT Rectovestibular fistula, Uterine transplantation in Mayer-Rokitansky-Kuster̈ -Hauser (MRKH) patients with absolute Vaginal atresia, uterine function infertility have added a new dimension and paradigm shift in the Cervicovaginal atresia, management of females born with rectovestibular fistula coexisting with vaginal agenesis. MRKH Syndrome, The author reviewed the relevant literature of this rare association, the popular and practical Vaginoplasty, Bowel vaginoplasty, classifications of genital malformations that the gynecologists use, the different vaginal Ecchietti vaginoplasty, reconstruction techniques, and try to know what shall serve best in this small cohort of Uterine transplantation, these patients lest they wish to go for uterine transplantation in future. VCUA classification, ESHRE/ESGE classification, AFC classification, Krickenbeck classification INTRODUCTION -

Background Briefing Document from the Consortium of Sponsors for The
BRIEFING BOOK FOR Pediatric Advisory Committee (PAC) Prepared by Alan Rogol, M.D., Ph.D. Prepared for Consortium of Sponsors Meeting Date: April 8th, 2019 TABLE OF CONTENTS LIST OF FIGURES ................................................... ERROR! BOOKMARK NOT DEFINED. LIST OF TABLES ...........................................................................................................................4 1. INTRODUCTION AND BACKGROUND FOR THE MEETING .............................6 1.1. INDICATION AND USAGE .......................................................................................6 2. SPONSOR CONSORTIUM PARTICIPANTS ............................................................6 2.1. TIMELINE FOR SPONSOR ENGAGEMENT FOR PEDIATRIC ADVISORY COMMITTEE (PAC): ............................................................................6 3. BACKGROUND AND RATIONALE .........................................................................7 3.1. INTRODUCTION ........................................................................................................7 3.2. PHYSICAL CHANGES OF PUBERTY ......................................................................7 3.2.1. Boys ..............................................................................................................................7 3.2.2. Growth and Pubertal Development ..............................................................................8 3.3. AGE AT ONSET OF PUBERTY.................................................................................9 3.4. -

Management of Vaginal Hypoplasia in Disorders of Sexual Development: Surgical and Non-Surgical Options
Sex Dev 2010;4:292–299 Published online: July 24, 2010 DOI: 10.1159/000316231 Management of Vaginal Hypoplasia in Disorders of Sexual Development: Surgical and Non-Surgical Options R. Deans M. Berra S.M. Creighton University College Hospital, Institute of Women’s Health, London , UK Key Words gardless of the vaginal reconstruction technique, patients Disorders of sexual development ؒ Surgery ؒ should be managed in a multidisciplinary team where there Vaginal hypoplasia is adequate emotional and psychological support available. Copyright © 2010 S. Karger AG, Basel Abstract Patients with disorders of sexual development (DSD) requir- Introduction ing vaginal reconstruction are complex and varied in their presentation. Enlargement procedures for vaginal hypo- Patients with disorders of sexual development (DSD) plasia include self-dilation therapy or surgical vaginoplasty. requiring vaginal reconstruction are complex and varied There are many vaginoplasty techniques described, and in their presentation. Enlargement procedures for vagi- each method has different risks and benefits. Reviewing the nal hypoplasia include self-dilation therapy or surgical literature on management options for vaginal hypoplasia, vaginoplasty. These interventions are offered to improve the results show a number of techniques available for the psychological and sexual outcomes. The concept of sur- creation of a neovagina. Studies are difficult to compare due gery for DSD conditions has become increasingly contro- to their heterogeneity, and the indications for surgery are versial in the last decade. Clinicians and patients have not always clear. Psychological support improves outcomes. become involved in the debate, with strong views on both There is a paucity of evidence to inform management re- sides of the fence, with minimal evidence to inform man- garding the optimum surgical technique to use, and long- agement. -
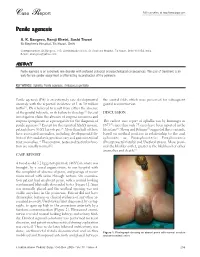
Case Report Full Text Online At
Case Report Full text online at http://www.jiaps.com Penile agenesis A. K. Bangroo, Ramji Khetri, Sashi Tiwari St Stephen's Hospital, Tis Hazari, Delhi Correspondence: AK Bangroo, 103, Administrative block, St. Stephens Hospital, Tis Hazari, Delhi-110054, India. E-mail: [email protected] ABSTRACT Penile agenesis is an extremely rare disorder with profound urological and psychological consequences. The goal of treatment is an early female gender assignment and feminizing reconstruction of the perineum. KEY WORDS: Aphallia, Penile agenesis, Ambiguous genitalia Penile agenesis (PA) is an extremely rare developmental the scrotal folds which were preserved for subsequent anomaly with the reported incidence of 1 in 30 million genital reconstruction. births[1]. PA is believed to result from either the absence of the genital tubercle, or its failure to develop.[2] Several DISCUSSION investigators claim the absence of corpora cavernosa and corpora spongiosum as a prerequisite for the diagnosis of The earliest case report of aphallia was by Imminger in penile agenesis.[3] Except for the reported XX-XY mosaic, 1853[2] since then only 75 cases have been reported in the patients have 46 XY karyotypes.[4] More than half of these literature[6]. Skoog and Belman[5] suggested three variants, have associated anomalies, including developmental de based on urethral position in relationship to the anal fects of the caudal axis, genitourinary and gastrointestinal sphincter, as: Postsphincteric; Presphincteric tract anomalies.[5] The scrotum, testes and testicular func (Prostatorectal fistula) and Urethral atresia. More proxi tion are usually normal[2]. mal the bladder outlet, greater is the likelihood of other anomalies and death.[5] CASE REPORT A two-day-old 3.2 kg genotypic male (46XY) neonate was brought, by a social organization, to our hospital with the complaint of absence of penis, and passage of meco nium mixed with urine through rectum. -

EAU Pocket Guidelines on Male Hypogonadism 2013
GUIDELINES ON MALE HYPOGONADISM G.R. Dohle (chair), S. Arver, C. Bettocchi, S. Kliesch, M. Punab, W. de Ronde Introduction Male hypogonadism is a clinical syndrome caused by andro- gen deficiency. It may adversely affect multiple organ func- tions and quality of life. Androgens play a crucial role in the development and maintenance of male reproductive and sexual functions. Low levels of circulating androgens can cause disturbances in male sexual development, resulting in congenital abnormalities of the male reproductive tract. Later in life, this may cause reduced fertility, sexual dysfunc- tion, decreased muscle formation and bone mineralisation, disturbances of fat metabolism, and cognitive dysfunction. Testosterone levels decrease as a process of ageing: signs and symptoms caused by this decline can be considered a normal part of ageing. However, low testosterone levels are also associated with several chronic diseases, and sympto- matic patients may benefit from testosterone treatment. Androgen deficiency increases with age; an annual decline in circulating testosterone of 0.4-2.0% has been reported. In middle-aged men, the incidence was found to be 6%. It is more prevalent in older men, in men with obesity, those with co-morbidities, and in men with a poor health status. Aetiology and forms Male hypogonadism can be classified in 4 forms: 1. Primary forms caused by testicular insufficiency. 2. Secondary forms caused by hypothalamic-pituitary dysfunction. 164 Male Hypogonadism 3. Late onset hypogonadism. 4. Male hypogonadism due to androgen receptor insensitivity. The main causes of these different forms of hypogonadism are highlighted in Table 1. The type of hypogonadism has to be differentiated, as this has implications for patient evaluation and treatment and enables identification of patients with associated health problems. -

A MRI Diagnosis of Congenital Urogenital Anomalies in 27 Years
Journal of Advances in Radiology and Medical Imaging Volume 4 | Issue 1 ISSN: 2456-5504 Case Report Open Access A MRI Diagnosis of Congenital Urogenital Anomalies in 27 Years Old Man D’Amato D*, Ranalli T, Tatulli D, Bocchinfuso F, Manenti G, Valente F and Bizzaglia M Diagnostic and Interventional Radiology, Policlinico Tor Vergata, University of Rome “Tor Vergata”, Rome, Italy *Corresponding author: D’Amato D, Diagnostic and Interventional Radiology, Policlinico Tor Vergata, University of Rome “Tor Vergata”, Viale Oxford 181, Rome, Italy, Tel: +393207034690, E-mail: [email protected] Citation: D’Amato D, Ranalli T, Tatulli D, Bocchinfuso F, Manenti G, et al. (2019) A MRI Diagnosis of Congenital Urogenital Anomalies in 27 Years Old Man. J Adv Radiol Med Image 4(1): 102 Received Date: April 04, 2019 Accepted Date: August 26, 2019 Published Date: August 28, 2019 Abstract Congenital anorchia is an uncommon clinical condition. Etiology and pathogenetic mechanisms are often unknown. Although some patients with anorchia present with ambiguous external genitalia or micropenis, most have a normal phenotype. XY Disorders of Sex Development classifications are numerous and success rate in establishing a precise diagnosis is far lower than in XX karyotype. We report the case of a young man, with 46 XY karyotype showing various uro-genital abnormalities. A definitive diagnosis was not established due to the complex clinical presentation. Ultrasonography and Magnetic Resonance Imaging techniques were useful tools in the definition of uro-genital anomalies and gonadal development in this complex scenario. Keywords: Anorchia; Cryptorchidism; Urogenital Anomalies; DSD; MRI List of abbreviations: MRI: Magnetic Resonance Imaging; US: Ultrasonography; DSD: Disorders of Sex Development, FSH: Follicle- Stimulating Hormone; HCG: Human Chorionic Gonadotropin; LH: Luteinizing Hormone; AMH Antimüllerian Hormone; LHRH LH- Releasing Hormone; SD: Standard Deviation Introduction The disorders of sexual differentiation constitute a challenging area for both diagnostic and therapeutic impact. -

The Genetic Basis for Skeletal Diseases
insight review articles The genetic basis for skeletal diseases Elazar Zelzer & Bjorn R. Olsen Harvard Medical School, Department of Cell Biology, 240 Longwood Avenue, Boston, Massachusetts 02115, USA (e-mail: [email protected]) We walk, run, work and play, paying little attention to our bones, their joints and their muscle connections, because the system works. Evolution has refined robust genetic mechanisms for skeletal development and growth that are able to direct the formation of a complex, yet wonderfully adaptable organ system. How is it done? Recent studies of rare genetic diseases have identified many of the critical transcription factors and signalling pathways specifying the normal development of bones, confirming the wisdom of William Harvey when he said: “nature is nowhere accustomed more openly to display her secret mysteries than in cases where she shows traces of her workings apart from the beaten path”. enetic studies of diseases that affect skeletal differentiation to cartilage cells (chondrocytes) or bone cells development and growth are providing (osteoblasts) within the condensations. Subsequent growth invaluable insights into the roles not only of during the organogenesis phase generates cartilage models individual genes, but also of entire (anlagen) of future bones (as in limb bones) or membranous developmental pathways. Different mutations bones (as in the cranial vault) (Fig. 1). The cartilage anlagen Gin the same gene may result in a range of abnormalities, are replaced by bone and marrow in a process called endo- and disease ‘families’ are frequently caused by mutations in chondral ossification. Finally, a process of growth and components of the same pathway. -

Clinical, Hormonal and Genetic Characteristics of Androgen Insensitivity Syndrome in 39 Chinese Patients Qingxu Liu, Xiaoqin Yin and Pin Li*
Liu et al. Reproductive Biology and Endocrinology (2020) 18:34 https://doi.org/10.1186/s12958-020-00593-0 RESEARCH Open Access Clinical, hormonal and genetic characteristics of androgen insensitivity syndrome in 39 Chinese patients Qingxu Liu, Xiaoqin Yin and Pin Li* Abstract Background: Abnormal androgen receptor (AR) genes can cause androgen insensitivity syndrome (AIS), and AIS can be classified into complete androgen insensitivity syndrome (CAIS), partial androgen insensitivity syndrome (PAIS) and mild AIS. We investigated the characteristics of clinical manifestations, serum sex hormone levels and AR gene mutations of 39 AIS patients, which provided deeper insight into this disease. Methods: We prospectively evaluated 39 patients with 46, XY disorders of sex development (46, XY DSD) who were diagnosed with AIS at the Department of Endocrinology of Shanghai Children’s Hospital from 2014 to 2019. We analysed clinical data from the patients including hormone levels and AR gene sequences. Furthermore, we screened the AR gene sequences of the 39 AIS patients to identify probable mutations. Results: The 39 AIS patients came from 37 different families; 19 of the patients presented CAIS, and 20 of them presented PAIS. The CAIS patients exhibited a higher cryptorchidism rate than the PAIS (100 and 55%, P = 0.001). There were no significant difference between the CAIS and PAIS groups regarding the levels of inhibin B (INHB), sex hormone-binding globulin (SHBG), basal luteinizing hormone (LH), testosterone (T), or basal dihydrotestosterone (DHT), the T:DHT ratio, DHT levels after human chorionic gonadotropin (HCG) stimulation or T levels after HCG stimulation. However, the hormone levels of AMH (P = 0.010), peak LH (P = 0.033), basal FSH (P = 0.009) and peak FSH (P = 0.033) showed significant differences between the CAIS group and the PAIS group. -

Hyperplasia (Growth Factors
Adaptations Robbins Basic Pathology Robbins Basic Pathology Robbins Basic Pathology Coagulation Robbins Basic Pathology Robbins Basic Pathology Homeostasis • Maintenance of a steady state Adaptations • Reversible functional and structural responses to physiologic stress and some pathogenic stimuli • New altered “steady state” is achieved Adaptive responses • Hypertrophy • Altered demand (muscle . hyper = above, more activity) . trophe = nourishment, food • Altered stimulation • Hyperplasia (growth factors, . plastein = (v.) to form, to shape; hormones) (n.) growth, development • Altered nutrition • Dysplasia (including gas exchange) . dys = bad or disordered • Metaplasia . meta = change or beyond • Hypoplasia . hypo = below, less • Atrophy, Aplasia, Agenesis . a = without . nourishment, form, begining Robbins Basic Pathology Cell death, the end result of progressive cell injury, is one of the most crucial events in the evolution of disease in any tissue or organ. It results from diverse causes, including ischemia (reduced blood flow), infection, and toxins. Cell death is also a normal and essential process in embryogenesis, the development of organs, and the maintenance of homeostasis. Two principal pathways of cell death, necrosis and apoptosis. Nutrient deprivation triggers an adaptive cellular response called autophagy that may also culminate in cell death. Adaptations • Hypertrophy • Hyperplasia • Atrophy • Metaplasia HYPERTROPHY Hypertrophy refers to an increase in the size of cells, resulting in an increase in the size of the organ No new cells, just larger cells. The increased size of the cells is due to the synthesis of more structural components of the cells usually proteins. Cells capable of division may respond to stress by undergoing both hyperrtophy and hyperplasia Non-dividing cell increased tissue mass is due to hypertrophy.