Microrefugia, Climate Change, and Conservation of Cedrus Atlantica In
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Timeline of Natural History
Timeline of natural history This timeline of natural history summarizes significant geological and Life timeline Ice Ages biological events from the formation of the 0 — Primates Quater nary Flowers ←Earliest apes Earth to the arrival of modern humans. P Birds h Mammals – Plants Dinosaurs Times are listed in millions of years, or Karo o a n ← Andean Tetrapoda megaanni (Ma). -50 0 — e Arthropods Molluscs r ←Cambrian explosion o ← Cryoge nian Ediacara biota – z ←Earliest animals o ←Earliest plants i Multicellular -1000 — c Contents life ←Sexual reproduction Dating of the Geologic record – P r The earliest Solar System -1500 — o t Precambrian Supereon – e r Eukaryotes Hadean Eon o -2000 — z o Archean Eon i Huron ian – c Eoarchean Era ←Oxygen crisis Paleoarchean Era -2500 — ←Atmospheric oxygen Mesoarchean Era – Photosynthesis Neoarchean Era Pong ola Proterozoic Eon -3000 — A r Paleoproterozoic Era c – h Siderian Period e a Rhyacian Period -3500 — n ←Earliest oxygen Orosirian Period Single-celled – life Statherian Period -4000 — ←Earliest life Mesoproterozoic Era H Calymmian Period a water – d e Ectasian Period a ←Earliest water Stenian Period -4500 — n ←Earth (−4540) (million years ago) Clickable Neoproterozoic Era ( Tonian Period Cryogenian Period Ediacaran Period Phanerozoic Eon Paleozoic Era Cambrian Period Ordovician Period Silurian Period Devonian Period Carboniferous Period Permian Period Mesozoic Era Triassic Period Jurassic Period Cretaceous Period Cenozoic Era Paleogene Period Neogene Period Quaternary Period Etymology of period names References See also External links Dating of the Geologic record The Geologic record is the strata (layers) of rock in the planet's crust and the science of geology is much concerned with the age and origin of all rocks to determine the history and formation of Earth and to understand the forces that have acted upon it. -

Museum of Economic Botany, Kew. Specimens Distributed 1901 - 1990
Museum of Economic Botany, Kew. Specimens distributed 1901 - 1990 Page 1 - https://biodiversitylibrary.org/page/57407494 15 July 1901 Dr T Johnson FLS, Science and Art Museum, Dublin Two cases containing the following:- Ackd 20.7.01 1. Wood of Chloroxylon swietenia, Godaveri (2 pieces) Paris Exibition 1900 2. Wood of Chloroxylon swietenia, Godaveri (2 pieces) Paris Exibition 1900 3. Wood of Melia indica, Anantapur, Paris Exhibition 1900 4. Wood of Anogeissus acuminata, Ganjam, Paris Exhibition 1900 5. Wood of Xylia dolabriformis, Godaveri, Paris Exhibition 1900 6. Wood of Pterocarpus Marsupium, Kistna, Paris Exhibition 1900 7. Wood of Lagerstremia parviflora, Godaveri, Paris Exhibition 1900 8. Wood of Anogeissus latifolia , Godaveri, Paris Exhibition 1900 9. Wood of Gyrocarpus jacquini, Kistna, Paris Exhibition 1900 10. Wood of Acrocarpus fraxinifolium, Nilgiris, Paris Exhibition 1900 11. Wood of Ulmus integrifolia, Nilgiris, Paris Exhibition 1900 12. Wood of Phyllanthus emblica, Assam, Paris Exhibition 1900 13. Wood of Adina cordifolia, Godaveri, Paris Exhibition 1900 14. Wood of Melia indica, Anantapur, Paris Exhibition 1900 15. Wood of Cedrela toona, Nilgiris, Paris Exhibition 1900 16. Wood of Premna bengalensis, Assam, Paris Exhibition 1900 17. Wood of Artocarpus chaplasha, Assam, Paris Exhibition 1900 18. Wood of Artocarpus integrifolia, Nilgiris, Paris Exhibition 1900 19. Wood of Ulmus wallichiana, N. India, Paris Exhibition 1900 20. Wood of Diospyros kurzii , India, Paris Exhibition 1900 21. Wood of Hardwickia binata, Kistna, Paris Exhibition 1900 22. Flowers of Heterotheca inuloides, Mexico, Paris Exhibition 1900 23. Leaves of Datura Stramonium, Paris Exhibition 1900 24. Plant of Mentha viridis, Paris Exhibition 1900 25. Plant of Monsonia ovata, S. -

EGP Sale Uruguay
Media Relations PRESS T +39 06 8305 5699 RELEASE F +39 06 8305 3771 [email protected] enelgreenpower.com ENEL GREEN POWER STEPS OUT OF URUGUAY THROUGH SALE OF 50 MW WIND FARM FOR 120 MILLION US DOLLARS • EGP closed the sale to Atlantica Yield of Enel Green Power Uruguay S.A., 100% owner of the Melowind plant • The transaction is part of Enel’s active portfolio management strategy, rotating assets to finance growth in strategic areas Rome, December 14th , 2018 – Enel Green Power S.p.A. (“EGP”) closed the sale to power company Atlantica Yield of its fully-owned subsidiary Enel Green Power Uruguay S.A. (“EGP Uruguay”), which owns through its project company Estrellada S.A. the 50 MW Melowind wind farm located in Cerro Largo, around 320 km away from Montevideo. EGP has sold its subsidiary in Uruguay for around 120 million US dollars, equal to the company’s Enterprise Value. The transaction is part of the disposal programme of non-core assets provided for in the Enel Group’s active portfolio management plan. This strategy allows for the reallocation of resources to areas with a greater growth margin and potential for the Group. The Melowind farm sells its electricity output to the state-owned power company UTE (Administración Nacional de Usinas y Trasmisiones Eléctricas), which manages the transmission, distribution and sale of electricity in Uruguay, under a 20-year power purchase agreement (PPA). Atlantica Yield plc owns a diversified portfolio of contracted renewable energy, efficient natural gas, electric transmission and water assets in North and South America, and certain markets in Europe, the Middle East and Africa (EMEA). -

Mafic Dyke Swarm, Brazil: Implications for Archean Supercratons
Michigan Technological University Digital Commons @ Michigan Tech Michigan Tech Publications 12-3-2018 Revisiting the paleomagnetism of the Neoarchean Uauá mafic dyke swarm, Brazil: Implications for Archean supercratons J. Salminen University of Helsinki E. P. Oliveira State University of Campinas, Brazil Elisa J. Piispa Michigan Technological University Aleksey Smirnov Michigan Technological University, [email protected] R. I. F. Trindade Universidade de Sao Paulo Follow this and additional works at: https://digitalcommons.mtu.edu/michigantech-p Part of the Geological Engineering Commons, and the Physics Commons Recommended Citation Salminen, J., Oliveira, E. P., Piispa, E. J., Smirnov, A., & Trindade, R. I. (2018). Revisiting the paleomagnetism of the Neoarchean Uauá mafic dyke swarm, Brazil: Implications for Archean supercratons. Precambrian Research, 329, 108-123. http://dx.doi.org/10.1016/j.precamres.2018.12.001 Retrieved from: https://digitalcommons.mtu.edu/michigantech-p/443 Follow this and additional works at: https://digitalcommons.mtu.edu/michigantech-p Part of the Geological Engineering Commons, and the Physics Commons Precambrian Research 329 (2019) 108–123 Contents lists available at ScienceDirect Precambrian Research journal homepage: www.elsevier.com/locate/precamres Revisiting the paleomagnetism of the Neoarchean Uauá mafic dyke swarm, T Brazil: Implications for Archean supercratons ⁎ J. Salminena,b, , E.P. Oliveirac, E.J. Piispad,e, A.V. Smirnovd,f, R.I.F. Trindadeg a Physics Department, University of Helsinki, P.O. Box -

Dead Sea Pollen Provides New Insights Into the Paleoenvironment of the Southern Levant During MIS 6ᅢ까タᅡモ5
Quaternary Science Reviews 188 (2018) 15e27 Contents lists available at ScienceDirect Quaternary Science Reviews journal homepage: www.elsevier.com/locate/quascirev Dead Sea pollen provides new insights into the paleoenvironment of the southern Levant during MIS 6e5 * Chunzhu Chen a, b, , Thomas Litt b a School of Geographic Science, Nantong University, Tongjingdadao 999, 226007 Nantong, China b Steinmann Institute for Geology, Mineralogy, and Paleontology, University of Bonn, Nussallee 8, 53115 Bonn, Germany article info abstract Article history: The paleoclimate of the southern Levant, especially during the last interglacial (LIG), is still under debate. Received 18 December 2017 Reliable paleovegetation information for this period, as independent evidence to the paleoenvironment, Received in revised form was still missing. In this study, we present a high-resolution pollen record encompassing 147e89 ka from 17 March 2018 the Dead Sea deep drilling core 5017-1A. The sediment profile is marked by alternations of laminated Accepted 18 March 2018 marl deposits and thick massive halite, indicating lake-level fluctuations. The pollen record suggests that steppe and desert components predominated in the Dead Sea surroundings during the whole investi- gated interval. The late penultimate glacial (147.3e130.9 ka) and early last glacial (115.5e89.1 ka) were Keywords: fi Last interglacial cool and relatively dry, with sub-humid conditions con ned to the mountains that sustained moderate Paleovegetation amounts of deciduous oaks. Prior to the LIG optimum, a prevalence of desert components and a Lake level concomitant increase in frost-sensitive pistachio trees demonstrate the occurrence of an arid initial Early modern human migration warming phase (130.9e124.2 ka). -

Precambrian Basement and Late Paleoproterozoic to Mesoproterozoic Tectonic Evolution of the SW Yangtze Block, South China
minerals Article Precambrian Basement and Late Paleoproterozoic to Mesoproterozoic Tectonic Evolution of the SW Yangtze Block, South China: Constraints from Zircon U–Pb Dating and Hf Isotopes Wei Liu 1,2,*, Xiaoyong Yang 1,*, Shengyuan Shu 1, Lei Liu 1 and Sihua Yuan 3 1 CAS Key Laboratory of Crust-Mantle Materials and Environments, University of Science and Technology of China, Hefei 230026, China; [email protected] (S.S.); [email protected] (L.L.) 2 Chengdu Center, China Geological Survey, Chengdu 610081, China 3 Department of Earthquake Science, Institute of Disaster Prevention, Langfang 065201, China; [email protected] * Correspondence: [email protected] (W.L.); [email protected] (X.Y.) Received: 27 May 2018; Accepted: 30 July 2018; Published: 3 August 2018 Abstract: Zircon U–Pb dating and Hf isotopic analyses are performed on clastic rocks, sedimentary tuff of the Dongchuan Group (DCG), and a diabase, which is an intrusive body from the base of DCG in the SW Yangtze Block. The results provide new constraints on the Precambrian basement and the Late Paleoproterozoic to Mesoproterozoic tectonic evolution of the SW Yangtze Block, South China. DCG has been divided into four formations from the bottom to the top: Yinmin, Luoxue, Heishan, and Qinglongshan. The Yinmin Formation, which represents the oldest rock unit of DCG, was intruded by a diabase dyke. The oldest zircon age of the clastic rocks from the Yinmin Formation is 3654 Ma, with "Hf(t) of −3.1 and a two-stage modeled age of 4081 Ma. Another zircon exhibits an age of 2406 Ma, with "Hf(t) of −20.1 and a two-stage modeled age of 4152 Ma. -

THE SILVICULTURE of TREES USED in BRITISH FORESTRY, 2ND EDITION This Page Intentionally Left Blank the Silviculture of Trees Used in British Forestry, 2Nd Edition
THE SILVICULTURE OF TREES USED IN BRITISH FORESTRY, 2ND EDITION This page intentionally left blank The Silviculture of Trees Used in British Forestry, 2nd Edition Peter Savill Former Reader in Silviculture University of Oxford With illustrations by Rosemary Wise CABI is a trading name of CAB International CABI CABI Nosworthy Way 38 Chauncey Street Wallingford Suite 1002 Oxfordshire OX10 8DE Boston, MA 02111 UK USA Tel: +44 (0)1491 832111 Tel: +1 800 552 3083 (toll free) Fax: +44 (0)1491 833508 Tel: +1 (0)617 395 4051 E-mail: [email protected] E-mail: [email protected] Website: www.cabi.org © Peter Savill 2013. All rights reserved. No part of this publication may be reproduced in any form or by any means, electronically, mechanically, by photocopying, recording or otherwise, without the prior permission of the copyright owners. A catalogue record for this book is available from the British Library, London, UK. Library of Congress Cataloging-in-Publication Data Savill, Peter S. The silviculture of trees used in British forestry / Peter Savill. -- 2nd ed. p. cm. Includes bibliographical references and index. ISBN 978-1-78064-026-6 (alk. paper) 1. Forests and forestry--Great Britain. 2. Trees--Great Britain. I. Title. SD391.S285 2013 634.90941--dc23 2012043421 ISBN-13: 978 1 78064 026 6 Commissioning editor: Vicki Bonham Editorial assistants: Emma McCann and Alexandra Lainsbury Production editor: Lauren Povey Typeset by SPi, Pondicherry, India. Printed and bound in the UK by the MPG Books Group. Contents Acknowledgements viii Introduction 1 ABIES -
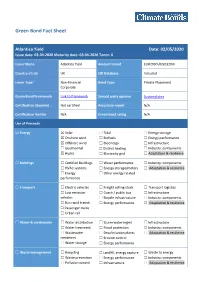
Atlantica Yield Date: 02/05/2020 Issue Date: 03-04-2020 Maturity Date: 03-04-2026 Tenor: 6
Green Bond Fact Sheet Atlantica Yield Date: 02/05/2020 Issue date: 03-04-2020 Maturity date: 03-04-2026 Tenor: 6 Issuer Name Atlantica Yield Amount Issued EUR290m/USD320m Country of risk UK CBI Database Included Issuer Type1 Non-Financial Bond Type Private Placement Corporate Green Bond Framework Link to framework Second party opinion Sustainalytics Certification Standard Not certified Assurance report N/A Certification Verifier N/A Green bond rating N/A Use of Proceeds ☒ Energy ☒ Solar ☐ Tidal ☐ Energy storage ☒ Onshore wind ☐ Biofuels ☐ Energy performance ☒ Offshore wind ☐ Bioenergy ☐ Infrastructure ☐ Geothermal ☐ District heating ☐ Industry: components ☒ Hydro ☐ Electricity grid ☐ Adaptation & resilience ☐ Buildings ☐ Certified Buildings ☐ Water performance ☐ Industry: components ☐ HVAC systems ☐ Energy storage/meters ☐ Adaptation & resilience ☐ Energy ☐ Other energy related performance ☐ Transport ☐ Electric vehicles ☐ Freight rolling stock ☐ Transport logistics ☐ Low emission ☐ Coach / public bus ☐ Infrastructure vehicles ☐ Bicycle infrastructure ☐ Industry: components ☐ Bus rapid transit ☐ Energy performance ☐ Adaptation & resilience ☐ Passenger trains ☐ Urban rail ☐ Water & wastewater ☐ Water distribution ☐ Storm water mgmt ☐ Infrastructure ☐ Water treatment ☐ Flood protection ☐ Industry: components ☐ Wastewater ☐ Desalinisation plants ☐ Adaptation & resilience treatment ☐ Erosion control ☐ Water storage ☐ Energy performance ☐ Waste management ☐ Recycling ☐ Landfill, energy capture ☐ Waste to energy ☐ Waste prevention ☐ Energy -

Assessment of How Natural Stand Structure for Narrow Endemic Cedrus Brevifolia Henry Supports Silvicultural Treatments for Its Sustainable Management
Assessment of How Natural Stand Structure for Narrow Endemic Cedrus brevifolia Henry Supports Silvicultural Treatments for ItsISSN Sustainable 1847-6481 Management. eISSN 1849-0891 ORIGINAL SCIENTIFIC PAPER DOI: https://doi.org/10.15177/seefor.21-09 Assessment of How Natural Stand Structure for Narrow Endemic Cedrus brevifolia Henry Supports Silvicultural Treatments for Its Sustainable Management Elias Milios1,*, Petros Petrou2, Kyriakos Pytharidis2, Andreas K. Christou2, Nicolas-George H. Eliades3 (1) Democritus University of Thrace, Department of Forestry and Management of the Citation: Milios E, Petrou P, Pytharidis Environment and Natural Resources, Pantazidou 193, GR-68200 Orestiada, Greece; (2) K, Christou AK, Eliades N-GH, 2021. Ministry of Agriculture, Rural Development and Environment, Department of Forests, Assessment of How Natural Stand Loukis Akritas 26, CY-1414 Nicosia, Cyprus; (3) Frederick University, Nature Conservation Structure for Narrow Endemic Cedrus Unit, P.O. Box 24729, CY-1303 Nicosia, Cyprus brevifolia Henry Supports Silvicultural Treatments for Its Sustainable *Correspondence: [email protected] Management. South-east Eur for 12(1): 21-34. https://doi.org/10.15177/ seefor.21-09. Received: 16 Feb 2021; Revised: 27 Apr 2021; Accepted: 14 May 2021; Published online: 19 Jun 2021 ABSTRACT Cedrus brevifolia Henry is a narrow endemic tree species of Cyprus flora. The objectives of this study are to develop silvicultural treatments for the conservation of the species formations based on the stand structure analysis ofC. brevifolia natural forest and to present the characteristics of the first application of the treatments through silvicultural interventions. Six structural types were distinguished in C. brevifolia formations in the study area located in the state forest of Paphos. -

Trees, Shrubs, and Perennials That Intrigue Me (Gymnosperms First
Big-picture, evolutionary view of trees and shrubs (and a few of my favorite herbaceous perennials), ver. 2007-11-04 Descriptions of the trees and shrubs taken (stolen!!!) from online sources, from my own observations in and around Greenwood Lake, NY, and from these books: • Dirr’s Hardy Trees and Shrubs, Michael A. Dirr, Timber Press, © 1997 • Trees of North America (Golden field guide), C. Frank Brockman, St. Martin’s Press, © 2001 • Smithsonian Handbooks, Trees, Allen J. Coombes, Dorling Kindersley, © 2002 • Native Trees for North American Landscapes, Guy Sternberg with Jim Wilson, Timber Press, © 2004 • Complete Trees, Shrubs, and Hedges, Jacqueline Hériteau, © 2006 They are generally listed from most ancient to most recently evolved. (I’m not sure if this is true for the rosids and asterids, starting on page 30. I just listed them in the same order as Angiosperm Phylogeny Group II.) This document started out as my personal landscaping plan and morphed into something almost unwieldy and phantasmagorical. Key to symbols and colored text: Checkboxes indicate species and/or cultivars that I want. Checkmarks indicate those that I have (or that one of my neighbors has). Text in blue indicates shrub or hedge. (Unfinished task – there is no text in blue other than this text right here.) Text in red indicates that the species or cultivar is undesirable: • Out of range climatically (either wrong zone, or won’t do well because of differences in moisture or seasons, even though it is in the “right” zone). • Will grow too tall or wide and simply won’t fit well on my property. -

Second Party Opinion on Atlantica's Green Finance Framework
Second-Party Opinion Atlantica Green Finance Framework Evaluation Summary Evaluation March 17, 2020 Sustainalytics is of the opinion that the Atlantica Green Finance Framework is credible date and impactful and aligns with the four core components of the Green Bond Principles (GBP) 2018 and Green Loan Principles (GLP) 2018. This assessment is based on the Issuer Brentford, following: Location United Kingdom The eligible category for the use of proceeds – Renewable energy, is aligned with those recognized by the GBP 2018 Report and GLP 2018. Sustainalytics considers that the eligible category will Sections lead to positive environmental impacts and advance the UN Sustainable Development Goals 7 – Affordable & Clean Energy. Introduction ................................................ 2 Atlantica’s ESG and Finance Sustainalytics’ Opinion .............................. 3 Teams will be responsible for project evaluation and selection. The Appendices ................................................ 7 ESG Team will select and recommend eligible projects to the Finance Team who will then approve the recommended projects. Sustainalytics considers the project selection process in line with market practice. For inquiries, contact the Sustainable Atlantica has an internal tracking Finance Solutions project team: system to monitor and account for the funds raised under the Evan Bruner framework. Atlantica is managing the proceeds in a portfolio approach. Project Manager Atlantica will strive, over time, to achieve a level of allocation for the [email protected] Eligible Green Portfolio that matches or exceeds the balance of net (+31) 20 205 00 27 proceeds from its outstanding Green Bonds. Any unallocated proceeds will be held temporally in cash, cash equivalents, or other forms of Jean-Claude Berthelot (Amsterdam) available short-term funding sources. -

The Americas 2014-2015
The Americas 2014-2015 ALASKA | CANADA & NEW ENGLAND | CARIBBEAN & PANAMA CANAL | SOUTH AMERICA CoNTENTS 2 EXPERIENCE 96 EXPLORE ASHORE Your World. Your Way.® Land Tour Series 16 TaSTE 107 HoTEL PROGRamS The Finest Cuisine at Sea Pre- & Post-Cruise Hotel Programs 28 VaLUE 110 SUITES & STATERoomS Best Value in Upscale Cruising 120 DECK PLANS 32 OCEANIA CLUB Rewarding Membership Privileges 124 AIR PROGRamS & INfoRmaTION 34 DESTINATION SPECIALISTS 126 CRUISE CALENdaR 38 CaRIbbEAN & PaNama CaNAL 128 EXPERIENCE OCEANIACRUISES.Com 60 SoUTH AMERICA 129 GENERAL INfoRmaTION 66 ALASKA 78 CaNada & NEW ENGLAND 86 TRANSOCEANIC VoyaGES Hubbard Glacier Alaskan king crab ON THE COVER Colorful Maine lobster buoys are used to mark the lobster traps of local fishermen. As tradition shows, each fisherman paints a unique color and design onto a buoy to identify its origin of ownership. POINTS OF DISTINCTION n FREE AIRFARE* on every voyage n Mid-size, elegant ships catering to The Americas just 684 or 1,250 guests 2014 - 2015 n Finest cuisine at sea, served in a variety of distinctive open-seating restaurants, at no additional charge NEW WORLD ADVENTURES | European and Asian explorers once risked everything n to discover the New World and tame this rugged frontier. Now you, on board Oceania Gourmet culinary program crafted by world-renowned Master Chef Cruises, can trace the routes of the ages along the New England and Canadian shores, Jacques Pépin transformed by autumn’s embrace, or through the astounding Panama Canal. European and n Spectacular port-intensive itineraries African cultures mixed with native traditions gave birth to the tango, the passionate dance of featuring overnight visits and extended Buenos Aires.