A Systematic Study of the Avian Family Fringillidae Based on the Structure of the Skull
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

In Argentina New Birding ‘Lodges’ in Argentina James Lowen
>> BIRDING SITES NEW BIRDING LoDGES IN ARGENTINA New birding ‘lodges’ in Argentina James Lowen Birders visiting Argentina tend to stay in hotels near but not at birding sites because the country lacks lodges of the type found elsewhere in the Neotropics. However, a few new establishments are bucking the trend and may deserve to be added to country’s traditional birding route. This article focuses on two of them and highlights a further six. Note: all photographs were taken at the sites featured in the article. Long-trained Nightjar Macropsalis forcipata, Posada Puerto Bemberg, Misiones, June 2009 (emilio White); there is a good stakeout near the posada neotropical birding 6 49 >> BIRDING SITES NEW BIRDING LoDGES IN ARGENTINA lthough a relatively frequent destination Posada Puerto Bemberg, for Neotropical birders, Argentina—unlike A most Neotropical countries—has relatively Misiones few sites such as lodges where visitors can Pretty much every tourist visiting Misiones bird and sleep in the same place. Fortunately, province in extreme north-east Argentina makes there are signs that this is changing, as estancia a beeline for Iguazú Falls, a leading candidate to owners build lodgings and offer ecotourism- become one of UNESCO’s ‘seven natural wonders related services. In this article, I give an of the world’. Birders are no different, but also overview of two such sites that are not currently spend time in the surrounding Atlantic Forest on the standard Argentine birding trail—but of the Parque Nacional de Iguazú. Although should be. Both offer good birding and stylish some birders stay in the national park’s sole accommodation in a beautiful setting, which may hotel, most day-trip the area from hotels in interest those with non-birding partners. -

Grasshopper Sparrow,Ammodramus Savannarum Pratensis
COSEWIC Assessment and Status Report on the Grasshopper Sparrow Ammodramus savannarum pratensis pratensis subspecies (Ammodramus savannarum pratensis) in Canada SPECIAL CONCERN 2013 COSEWIC status reports are working documents used in assigning the status of wildlife species suspected of being at risk. This report may be cited as follows: COSEWIC. 2013. COSEWIC assessment and status report on the Grasshopper Sparrow pratensis subspecies Ammodramus savannarum pratensis in Canada. Committee on the Status of Endangered Wildlife in Canada. Ottawa. ix + 36 pp. (www.registrelep- sararegistry.gc.ca/default_e.cfm). Production note: COSEWIC acknowledges Carl Savignac for writing the status report on the Grasshopper Sparrow pratensis subspecies, Ammodramus savannarum pratensis in Canada, prepared with the financial support of Environment Canada. This report was overseen and edited by Marty Leonard, Co-chair of the COSEWIC Birds Specialist Subcommittee. For additional copies contact: COSEWIC Secretariat c/o Canadian Wildlife Service Environment Canada Ottawa, ON K1A 0H3 Tel.: 819-953-3215 Fax: 819-994-3684 E-mail: COSEWIC/[email protected] http://www.cosewic.gc.ca Également disponible en français sous le titre Ếvaluation et Rapport de situation du COSEPAC sur le Bruant sauterelle de la sous- espèce de l’Est (Ammodramus savannarum pratensis) au Canada. Cover illustration/photo: Grasshopper Sparrow pratensis subspecies — photo by Jacques Bouvier. Her Majesty the Queen in Right of Canada, 2014. Catalogue No. CW69-14/681-2014E-PDF ISBN 978-1-100-23548-6 Recycled paper COSEWIC Assessment Summary Assessment Summary – November 2013 Common name Grasshopper Sparrow - pratensis subspecies Scientific name Ammodramus savannarum pratensis Status Special Concern Reason for designation In Canada, this grassland bird is restricted to southern Ontario and southwestern Quebec. -

Proposal 2017-C-15 Below)
AOS Classification Committee – North and Middle America Proposal Set 2017-C 15 March 2017 No. Page Title 01 02 Revise the linear sequence of genera in Fringillidae, and transfer Serinus mozambicus to Crithagra 02 09 Split Brown Creeper (Certhia americana) into two species 03 16 Transfer Violet-bellied Hummingbird from Damophila to Juliamyia 04 18 Elevate Colaptes auratus mexicanoides to species rank 05 23 Split Nashville Warbler (Oreothlypis ruficapilla) into two species 06 26 Adopt new English names for Melozone biarcuata and Melozone cabanisi 07 29 Lump Thayer’s Gull (Larus thayeri) with Iceland Gull (Larus glaucoides) 08 43 Change the spelling of the English names of Le Conte’s Thrasher (Toxostoma lecontei) and Le Conte’s Sparrow (Ammodramus leconteii) 09 46 Add Common Scoter (Melanitta nigra) to the Main List 10 49 Add Blyth’s Reed Warbler (Acrocephalus dumetorum) to the Main List 11 52 Add Chatham Albatross (Thalassarche eremita) to the Main List 12 55 Add Red-legged Honeycreeper (Cyanerpes cyaneus) to the U.S. list 13 57 Add nine species recorded from Greenland to the Main List 14 68 Split Bell’s Vireo (Vireo bellii) into two species 1 2017-C-1 N&MA Classification Committee pp. 658-679 Revise the linear sequence of genera in Fringillidae, and transfer Serinus mozambicus to Crithagra Background: In the past decade, several phylogenetic papers have elucidated relationships within the Fringillidae (Arnaiz-Villena et al. 2007, 2008, Nguembock et al. 2009, Lerner et al. 2011, Zuccon et al. 2012). NACC already has taken a series of actions (reviewed below) based on this research. -

Tennessee Fish Species
The Angler’s Guide To TennesseeIncluding Aquatic Nuisance SpeciesFish Published by the Tennessee Wildlife Resources Agency Cover photograph Paul Shaw Graphics Designer Raleigh Holtam Thanks to the TWRA Fisheries Staff for their review and contributions to this publication. Special thanks to those that provided pictures for use in this publication. Partial funding of this publication was provided by a grant from the United States Fish & Wildlife Service through the Aquatic Nuisance Species Task Force. Tennessee Wildlife Resources Agency Authorization No. 328898, 58,500 copies, January, 2012. This public document was promulgated at a cost of $.42 per copy. Equal opportunity to participate in and benefit from programs of the Tennessee Wildlife Resources Agency is available to all persons without regard to their race, color, national origin, sex, age, dis- ability, or military service. TWRA is also an equal opportunity/equal access employer. Questions should be directed to TWRA, Human Resources Office, P.O. Box 40747, Nashville, TN 37204, (615) 781-6594 (TDD 781-6691), or to the U.S. Fish and Wildlife Service, Office for Human Resources, 4401 N. Fairfax Dr., Arlington, VA 22203. Contents Introduction ...............................................................................1 About Fish ..................................................................................2 Black Bass ...................................................................................3 Crappie ........................................................................................7 -
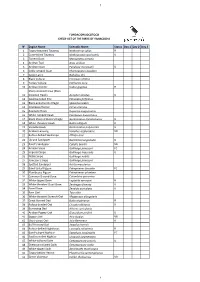
N° English Name Scientific Name Status Day 1
1 FUNDACIÓN JOCOTOCO CHECK-LIST OF THE BIRDS OF YANACOCHA N° English Name Scientific Name Status Day 1 Day 2 Day 3 1 Tawny-breasted Tinamou Nothocercus julius R 2 Curve-billed Tinamou Nothoprocta curvirostris U 3 Torrent Duck Merganetta armata 4 Andean Teal Anas andium 5 Andean Guan Penelope montagnii U 6 Sickle-winged Guan Chamaepetes goudotii 7 Cattle Egret Bubulcus ibis 8 Black Vulture Coragyps atratus 9 Turkey Vulture Cathartes aura 10 Andean Condor Vultur gryphus R Sharp-shinned Hawk (Plain- 11 breasted Hawk) Accipiter striatus U 12 Swallow-tailed Kite Elanoides forficatus 13 Black-and-chestnut Eagle Spizaetus isidori 14 Cinereous Harrier Circus cinereus 15 Roadside Hawk Rupornis magnirostris 16 White-rumped Hawk Parabuteo leucorrhous 17 Black-chested Buzzard-Eagle Geranoaetus melanoleucus U 18 White-throated Hawk Buteo albigula R 19 Variable Hawk Geranoaetus polyosoma U 20 Andean Lapwing Vanellus resplendens VR 21 Rufous-bellied Seedsnipe Attagis gayi 22 Upland Sandpiper Bartramia longicauda R 23 Baird's Sandpiper Calidris bairdii VR 24 Andean Snipe Gallinago jamesoni FC 25 Imperial Snipe Gallinago imperialis U 26 Noble Snipe Gallinago nobilis 27 Jameson's Snipe Gallinago jamesoni 28 Spotted Sandpiper Actitis macularius 29 Band-tailed Pigeon Patagoienas fasciata FC 30 Plumbeous Pigeon Patagioenas plumbea 31 Common Ground-Dove Columbina passerina 32 White-tipped Dove Leptotila verreauxi R 33 White-throated Quail-Dove Zentrygon frenata U 34 Eared Dove Zenaida auriculata U 35 Barn Owl Tyto alba 36 White-throated Screech-Owl Megascops -

RATES of KARYOTYPIC EVOLUTION in ESTRILDID FINCHES DIFFER BETWEEN 4 ISLAND and CONTINENTAL CLADES 5 6 Daniel M
bioRxiv preprint doi: https://doi.org/10.1101/013987; this version posted January 19, 2015. The copyright holder for this preprint (which was not certified by peer review) is the author/funder, who has granted bioRxiv a license to display the preprint in perpetuity. It is made available under aCC-BY-NC-ND 4.0 International license. 1 1 2 3 RATES OF KARYOTYPIC EVOLUTION IN ESTRILDID FINCHES DIFFER BETWEEN 4 ISLAND AND CONTINENTAL CLADES 5 6 Daniel M. Hooper1,2 and Trevor D. Price3 7 8 1Commitee on Evolutionary Biology, University of Chicago, Chicago, Illinois 60637 9 2 E-mail: [email protected] 10 3Department of Ecology and Evolution, University of Chicago, Chicago, Illinois 60637 11 12 13 Sunday, January 18, 2015 14 15 16 Running head: Chromosome inversions in finches 17 18 19 20 21 22 23 24 25 26 27 28 29 30 31 32 33 34 bioRxiv preprint doi: https://doi.org/10.1101/013987; this version posted January 19, 2015. The copyright holder for this preprint (which was not certified by peer review) is the author/funder, who has granted bioRxiv a license to display the preprint in perpetuity. It is made available under aCC-BY-NC-ND 4.0 International license. 2 35 Reasons why chromosomal rearrangements spread to fixation and frequently distinguish 36 related taxa remain poorly understood. We used cytological descriptions of karyotype to 37 identify large pericentric inversions between species of Estrildid finches (family 38 Estrildidae) and a time-dated phylogeny to assess the genomic, geographic, and 39 phylogenetic context of karyotype evolution in this group. -

L O U I S I a N A
L O U I S I A N A SPARROWS L O U I S I A N A SPARROWS Written by Bill Fontenot and Richard DeMay Photography by Greg Lavaty and Richard DeMay Designed and Illustrated by Diane K. Baker What is a Sparrow? Generally, sparrows are characterized as New World sparrows belong to the bird small, gray or brown-streaked, conical-billed family Emberizidae. Here in North America, birds that live on or near the ground. The sparrows are divided into 13 genera, which also cryptic blend of gray, white, black, and brown includes the towhees (genus Pipilo), longspurs hues which comprise a typical sparrow’s color (genus Calcarius), juncos (genus Junco), and pattern is the result of tens of thousands of Lark Bunting (genus Calamospiza) – all of sparrow generations living in grassland and which are technically sparrows. Emberizidae is brushland habitats. The triangular or cone- a large family, containing well over 300 species shaped bills inherent to most all sparrow species are perfectly adapted for a life of granivory – of crushing and husking seeds. “Of Louisiana’s 33 recorded sparrows, Sparrows possess well-developed claws on their toes, the evolutionary result of so much time spent on the ground, scratching for seeds only seven species breed here...” through leaf litter and other duff. Additionally, worldwide, 50 of which occur in the United most species incorporate a substantial amount States on a regular basis, and 33 of which have of insect, spider, snail, and other invertebrate been recorded for Louisiana. food items into their diets, especially during Of Louisiana’s 33 recorded sparrows, Opposite page: Bachman Sparrow the spring and summer months. -

Phylogeography of Finches and Sparrows
In: Animal Genetics ISBN: 978-1-60741-844-3 Editor: Leopold J. Rechi © 2009 Nova Science Publishers, Inc. Chapter 1 PHYLOGEOGRAPHY OF FINCHES AND SPARROWS Antonio Arnaiz-Villena*, Pablo Gomez-Prieto and Valentin Ruiz-del-Valle Department of Immunology, University Complutense, The Madrid Regional Blood Center, Madrid, Spain. ABSTRACT Fringillidae finches form a subfamily of songbirds (Passeriformes), which are presently distributed around the world. This subfamily includes canaries, goldfinches, greenfinches, rosefinches, and grosbeaks, among others. Molecular phylogenies obtained with mitochondrial DNA sequences show that these groups of finches are put together, but with some polytomies that have apparently evolved or radiated in parallel. The time of appearance on Earth of all studied groups is suggested to start after Middle Miocene Epoch, around 10 million years ago. Greenfinches (genus Carduelis) may have originated at Eurasian desert margins coming from Rhodopechys obsoleta (dessert finch) or an extinct pale plumage ancestor; it later acquired green plumage suitable for the greenfinch ecological niche, i.e.: woods. Multicolored Eurasian goldfinch (Carduelis carduelis) has a genetic extant ancestor, the green-feathered Carduelis citrinella (citril finch); this was thought to be a canary on phonotypical bases, but it is now included within goldfinches by our molecular genetics phylograms. Speciation events between citril finch and Eurasian goldfinch are related with the Mediterranean Messinian salinity crisis (5 million years ago). Linurgus olivaceus (oriole finch) is presently thriving in Equatorial Africa and was included in a separate genus (Linurgus) by itself on phenotypical bases. Our phylograms demonstrate that it is and old canary. Proposed genus Acanthis does not exist. Twite and linnet form a separate radiation from redpolls. -

Download Trip Report
SOUTH AFRICA: KRUGER NATIONAL PARK AND ESCARPMENT SET DEPARTURE TRIP REPORT 29 SEPTEMBER – 7 OCTOBER 2019 By Jason Boyce Kruger National Park is a great location to see many of the endangered Vulture species that we have in South Africa. Here is the Critically Endangered (IUCN) White-headed Vulture. www.birdingecotours.com [email protected] 2 | T R I P R E P O R T Kruger National Park and Escarpment September 2019 Overview This is one of my personal favorite areas to go birding in South Africa. The escarpment regions hold a variety of different habitats and can produce some really top birds, while the reputation of Kruger National Park speaks for itself. We spent a couple of days birding the grasslands of Dullstroom, the escarpment forests near Graskop, and the rocky landscapes through the J. G. Strijdom Tunnel. Top birds here included Cape Eagle Owl, Blue Crane, Gurney’s Sugarbird, Narina Trogon, and Knysna Turaco, not to mention the pair of African Finfoots seen in the Wilge River Valley en route to Dullstroom. Kruger National Park delivered right from day 1 with Dark Chanting Goshawk, White-headed Vulture, and Greater Painted Snipe around Letaba. We worked our way south from Letaba Rest Camp all the way to Berg en Dal Rest Camp over the next few days, with Satara Rest Camp producing Martial Eagle, the magnificent Saddle-billed Stork, and a large pride of Lions with cubs on the famous S100. Berg en Dal Rest Camp was great for birding, once again producing Retz’s Helmetshrike, Purple-banded Sunbird, African Barred Owlet, Purple-crested Turaco, and Bearded Scrub Robin. -

Dacninae Species Tree, Part I
Dacninae I: Nemosiini, Conirostrini, & Diglossini Hooded Tanager, Nemosia pileata Cherry-throated Tanager, Nemosia rourei Nemosiini Blue-backed Tanager, Cyanicterus cyanicterus White-capped Tanager, Sericossypha albocristata Scarlet-throated Tanager, Sericossypha loricata Bicolored Conebill, Conirostrum bicolor Pearly-breasted Conebill, Conirostrum margaritae Chestnut-vented Conebill, Conirostrum speciosum Conirostrini White-eared Conebill, Conirostrum leucogenys Capped Conebill, Conirostrum albifrons Giant Conebill, Conirostrum binghami Blue-backed Conebill, Conirostrum sitticolor White-browed Conebill, Conirostrum ferrugineiventre Tamarugo Conebill, Conirostrum tamarugense Rufous-browed Conebill, Conirostrum rufum Cinereous Conebill, Conirostrum cinereum Stripe-tailed Yellow-Finch, Pseudochloris citrina Gray-hooded Sierra Finch, Phrygilus gayi Patagonian Sierra Finch, Phrygilus patagonicus Peruvian Sierra Finch, Phrygilus punensis Black-hooded Sierra Finch, Phrygilus atriceps Gough Finch, Rowettia goughensis White-bridled Finch, Melanodera melanodera Yellow-bridled Finch, Melanodera xanthogramma Inaccessible Island Finch, Nesospiza acunhae Nightingale Island Finch, Nesospiza questi Wilkins’s Finch, Nesospiza wilkinsi Saffron Finch, Sicalis flaveola Grassland Yellow-Finch, Sicalis luteola Orange-fronted Yellow-Finch, Sicalis columbiana Sulphur-throated Finch, Sicalis taczanowskii Bright-rumped Yellow-Finch, Sicalis uropigyalis Citron-headed Yellow-Finch, Sicalis luteocephala Patagonian Yellow-Finch, Sicalis lebruni Greenish Yellow-Finch, -

Predation on Vertebrates by Neotropical Passerine Birds Leonardo E
Lundiana 6(1):57-66, 2005 © 2005 Instituto de Ciências Biológicas - UFMG ISSN 1676-6180 Predation on vertebrates by Neotropical passerine birds Leonardo E. Lopes1,2, Alexandre M. Fernandes1,3 & Miguel Â. Marini1,4 1 Depto. de Biologia Geral, Instituto de Ciências Biológicas, Universidade Federal de Minas Gerais, 31270-910, Belo Horizonte, MG, Brazil. 2 Current address: Lab. de Ornitologia, Depto. de Zoologia, Instituto de Ciências Biológicas, Universidade Federal de Minas Gerais, Av. Antônio Carlos, 6627, Pampulha, 31270-910, Belo Horizonte, MG, Brazil. E-mail: [email protected]. 3 Current address: Coleções Zoológicas, Aves, Instituto Nacional de Pesquisas da Amazônia, Avenida André Araújo, 2936, INPA II, 69083-000, Manaus, AM, Brazil. E-mail: [email protected]. 4 Current address: Lab. de Ornitologia, Depto. de Zoologia, Instituto de Biologia, Universidade de Brasília, 70910-900, Brasília, DF, Brazil. E-mail: [email protected] Abstract We investigated if passerine birds act as important predators of small vertebrates within the Neotropics. We surveyed published studies on bird diets, and information on labels of museum specimens, compiling data on the contents of 5,221 stomachs. Eighteen samples (0.3%) presented evidence of predation on vertebrates. Our bibliographic survey also provided records of 203 passerine species preying upon vertebrates, mainly frogs and lizards. Our data suggest that vertebrate predation by passerines is relatively uncommon in the Neotropics and not characteristic of any family. On the other hand, although rare, the ability to prey on vertebrates seems to be widely distributed among Neotropical passerines, which may respond opportunistically to the stimulus of a potential food item. -

The Relationships of the Hawaiian Honeycreepers (Drepaninini) As Indicated by Dna-Dna Hybridization
THE RELATIONSHIPS OF THE HAWAIIAN HONEYCREEPERS (DREPANININI) AS INDICATED BY DNA-DNA HYBRIDIZATION CH^RrES G. SIBLEY AND Jo• E. AHLQUIST Departmentof Biologyand PeabodyMuseum of Natural History, Yale University, New Haven, Connecticut 06511 USA ABSTRACT.--Twenty-twospecies of Hawaiian honeycreepers(Fringillidae: Carduelinae: Drepaninini) are known. Their relationshipsto other groups of passefineswere examined by comparing the single-copyDNA sequencesof the Apapane (Himationesanguinea) with those of 5 speciesof carduelinefinches, 1 speciesof Fringilla, 15 speciesof New World nine- primaried oscines(Cardinalini, Emberizini, Thraupini, Parulini, Icterini), and members of 6 other families of oscines(Turdidae, Monarchidae, Dicaeidae, Sylviidae, Vireonidae, Cor- vidae). The DNA-DNA hybridization data support other evidence indicating that the Hawaiian honeycreepersshared a more recent common ancestorwith the cardue!ine finches than with any of the other groupsstudied and indicate that this divergenceoccurred in the mid-Miocene, 15-20 million yr ago. The colonizationof the Hawaiian Islandsby the ancestralspecies that radiated to produce the Hawaiian honeycreeperscould have occurredat any time between 20 and 5 million yr ago. Becausethe honeycreeperscaptured so many ecologicalniches, however, it seemslikely that their ancestor was the first passefine to become established in the islands and that it arrived there at the time of, or soon after, its separationfrom the carduelinelineage. If so, this colonist arrived before the present islands from Hawaii to French Frigate Shoal were formed by the volcanic"hot-spot" now under the island of Hawaii. Therefore,the ancestral drepaninine may have colonizedone or more of the older Hawaiian Islandsand/or Emperor Seamounts,which also were formed over the "hot-spot" and which reachedtheir present positions as the result of tectonic crustal movement.