DNA Aneuploidy Study for Early Detection of Chromosomal
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Phenotype Manifestations of Polysomy X at Males
PHENOTYPE MANIFESTATIONS OF POLYSOMY X AT MALES Amra Ćatović* &Centre for Human Genetics, Faculty of Medicine, University of Sarajevo, Čekaluša , Sarajevo, Bosnia and Herzegovina * Corresponding author Abstract Klinefelter Syndrome is the most frequent form of male hypogonadism. It is an endocrine disorder based on sex chromosome aneuploidy. Infertility and gynaecomastia are the two most common symptoms that lead to diagnosis. Diagnosis of Klinefelter syndrome is made by karyotyping. Over years period (-) patients have been sent to “Center for Human Genetics” of Faculty of Medicine in Sarajevo from diff erent medical centres within Federation of Bosnia and Herzegovina with diagnosis suspecta Klinefelter syndrome, azoo- spermia, sterilitas primaria and hypogonadism for cytogenetic evaluation. Normal karyotype was found in (,) subjects, and karyotype was changed in (,) subjects. Polysomy X was found in (,) examinees. Polysomy X was expressed at the age of sexual maturity in the majority of the cases. Our results suggest that indication for chromosomal evaluation needs to be established at a very young age. KEY WORDS: polysomy X, hypogonadism, infertility Introduction Structural changes in gonosomes (X and Y) cause different distribution of genes, which may be exhibited in various phenotypes. Numerical aberrations of gonosomes have specific pattern of phenotype characteristics, which can be classified as clini- cal syndrome. Incidence of gonosome aberrations in males is / male newborn (). Klinefelter syndrome is the most common chromosomal disorder associated with male hypogonadism. According to different authors incidence is / male newborns (), /- (), and even / (). Very high incidence indicates that the zygotes with Klinefelter syndrome are more vital than those with other chromosomal aberrations. BOSNIAN JOURNAL OF BASIC MEDICAL SCIENCES 2008; 8 (3): 287-290 AMRA ĆATOVIĆ: PHENOTYPE MANIFESTATIONS OF POLYSOMY X AT MALES In , Klinefelter et al. -

Esmo Advanced Course on Ntrk Gene Fusion
ESMO ADVANCED COURSE ON NTRK GENE FUSION Mechanisms of gene fusion, fusion partners and consequences in oncogenesis Description, Structure and function of TRK and NTRK in ontogenesis Mehdi Brahmi Lyon, 13-14 September 2019 DISCLOSURE OF INTEREST No conflict of interest 1. Mechanisms of gene fusion 2. Gene fusion partners 3. Consequences in oncogenesis INTRODUCTION • Gene fusions (chromosomal rearrangements) • Juxtapositioning of 2 previously independent genes • From 2 nonhomologous chromosomes • Hybrid genes Translation of deregulated and/or chimeric protein • Most common type of structural chromosomal abnormalities • Not exclusive to cancer cells • Screening of cells from developing embryos • Translocations in 0.7 per 1000 live births = de novo • +/- associated to developmental abnormalities in some cases (unbalanced translocations) • Particularly common in cancer cells • Total number of gene fusions > 10 000 • > 90% identified in the past 5 years (deep-sequencing) • Listed in databases • COSMIC; http://www.sanger.ac.uk/genetics/CGP/cosmic/; dbCRID; http://dbcrid.biolead.org GENOMIC ALTERATIONS IN CANCER Drivers vs Passengers • High rate of chromosomal rearrangements in cancer • Most solid tumors • Changes in chromosome number (aneuploidy) • Deletions, inversions, translocations • Other genetic abnormalities • Drivers or Passengers • Passengers > Drivers • Alterations in non coding regions • Easy survival because mutations (TP53, etc…) • Statistical methods to identify “driver genes” • Frequency, Reccurence • Predicted effects « DRIVER -

Sperm Aneuploidy and DNA Fragmentation in Unexplained
Esquerré-Lamare et al. Basic and Clinical Andrology (2018) 28:4 https://doi.org/10.1186/s12610-018-0070-6 RESEARCH ARTICLE Open Access Sperm aneuploidy and DNA fragmentation in unexplained recurrent pregnancy loss: a multicenter case-control study Camille Esquerré-Lamare1,2, Marie Walschaerts1,2, Lucie Chansel Debordeaux3, Jessika Moreau1,2, Florence Bretelle4, François Isus1,5, Gilles Karsenty6, Laetitia Monteil7, Jeanne Perrin8,9, Aline Papaxanthos-Roche3 and Louis Bujan1,2* Abstract Background: Recurrent pregnancy loss (RPL) is defined as the loss of at least three pregnancies in the first trimester. Although the most common cause is embryo aneuploidy, and despite female checkup and couple karyotyping, in about 50% of cases RPL remain unexplained. Male implication has little been investigated and results are discordant. In this context, we conducted a multi-center prospective case-control study to investigate male gamete implication in unexplained RPL. Methods: A total of 33 cases and 27 controls were included from three university hospitals. We investigated environmental and family factors with a detailed questionnaire and andrological examination, sperm characteristics, sperm DNA/chromatin status using the sperm chromatin structure assay (SCSA) and terminal deoxynucleotidyl transferase dUTP nick end labeling (TUNEL) and sperm aneuploidy using fluorescence in situ hybridization (FISH). The Mann-Whitney test and the Wilcoxon or Fisher exact tests were used. A non-parametric Spearman correlation was performed in order to analyze the relationship between various sperm parameters and FISH and sperm DNA fragmentation results. Results: We found significant differences between cases and controls in time to conceive, body mass index (BMI), family history of infertility and living environment. -

Sex Chromosome Aneuploidies
7 Sex Chromosome Aneuploidies Eliona Demaliaj1, Albana Cerekja2 and Juan Piazze3 1Department of Obstetric-Gynecology, Faculty of Medicine, University of Tirana Hospital "Mbreteresha Geraldine", Tirane 2Gynecology and Obstetrics Ultrasound Division, ASL Roma B, Rome 3Ultrasound Division, Ospedale di Ceprano/Ospedale SS. Trinità di Sora, Frosinone 1Albania 2,3Italy 1. Introduction Sex chromosome aneuploidy is defined as a numeric abnormality of an X or Y chromosome, with addition or loss of an entire X or Y chromosome. Sex chromosome mosaicism, in which one or more populations of cells have lost or gained a sex chromosome, also is common. The most commonly occurring sex chromosome mosaic karyotypes include 45,X/46XX, 46XX/47,XXX, and 46,XY/47,XXY. Less frequent are those sex chromosome abnormalities where addition of more than one sex chromosome or a structural variant of an X or Y chromosome occur. The X chromosome is one of the two sex-determining chromosomes in many animal species, including mammals and is common in both males and females. It is a part of the XY and X0 sex-determination system. The X chromosome in humans represents about 2000 out of 20,000 - 25,0000 genes. Normal human females have 2 X-chromosomes (XX), for a total of nearly 4000 "sex-tied" genes (many of which have nothing to do with sex, other than being attached to the chromosome that is believed to cause sexual bimorphism (or polymorphism if you count more rare variations). Men have, depending on the study, 1400-1900 fewer genes, as the Y chromosome is thought to have only the remaining genes down from an estimated 1438 -~2000 (Graves 2004). -

Rare Double Aneuploidy in Down Syndrome (Down- Klinefelter Syndrome)
Research Article Journal of Molecular and Genetic Volume 14:2, 2020 DOI: 10.37421/jmgm.2020.14.444 Medicine ISSN: 1747-0862 Open Access Rare Double Aneuploidy in Down Syndrome (Down- Klinefelter Syndrome) Al-Buali Majed J1*, Al-Nahwi Fawatim A2, Al-Nowaiser Naziha A2, Al-Ali Rhaya A2, Al-Khamis Abdullah H2 and Al-Bahrani Hassan M2 1Deputy Chairman of Medical Genetic Unite, Pediatrics Department, Maternity Children Hospital Al-hassa, Hofuf, Saudi Arabia 2Pediatrics Resident, Pediatrics Department, Maternity Children Hospital Alhassa, Hofuf, Saudi Arabia Abstract Background: The chromosomal aneuploidy described as Cytogenetic condition characterized by abnormality in numbers of the chromosome. Aneuploid patient either trisomy or monosomy, can occur in both sex chromosomes as well as autosome chromosomes. However, double aneuploidies involving both sex and autosome chromosomes relatively a rare phenomenon. In present study, we reported a double aneuploidy (Down-Klinefelter syndrome) in infant from Saudi Arabia. Materials and Methods: In the present investigation, chromosomal analysis (standard chromosomal karyotyping) and fluorescence in situ hybridization (FISH) were performed according to the standard protocols. Results: Here, we report a single affected individual (boy) having Saudi origin, suffering from double chromosomal aneuploidy. The main presenting complaint is the obvious dysmorphic features suggesting Down syndrome. Chromosomal analysis and FISH revealed 48,XXY,+21, show the presence of three copies of chromosome 21, two copies of X chromosome and one copy of Y chromosome chromosomes. Conclusion: Patients with Down syndrome must be tested for other associated sex chromosome aneuploidies. Hence, proper diagnosis is needed for proper management and the cytogenetic tests should be performed as the first diagnostic approach. -

Double Aneuploidy in Down Syndrome
Chapter 6 Double Aneuploidy in Down Syndrome Fatma Soylemez Additional information is available at the end of the chapter http://dx.doi.org/10.5772/60438 Abstract Aneuploidy is the second most important category of chromosome mutations relat‐ ing to abnormal chromosome number. It generally arises by nondisjunction at ei‐ ther the first or second meiotic division. However, the existence of two chromosomal abnormalities involving both autosomal and sex chromosomes in the same individual is relatively a rare phenomenon. The underlying mechanism in‐ volved in the formation of double aneuploidy is not well understood. Parental ori‐ gin is studied only in a small number of cases and both nondisjunctions occurring in a single parent is an extremely rare event. This chapter reviews the characteristics of double aneuploidies in Down syndrome have been discussed in the light of the published reports. Keywords: Double aneuploidy, Down Syndrome, Klinefelter Syndrome, Chromo‐ some abnormalities 1. Introduction With the discovery in 1956 that the correct chromosome number in humans is 46, the new area of clinical cytogenetic began its rapid growth. Several major chromosomal syndromes with altered numbers of chromosomes were reported, such as Down syndrome (trisomy 21), Turner syndrome (45,X) and Klinefelter syndrome (47,XXY). Since then it has been well established that chromosome abnormalities contribute significantly to genetic disease resulting in reproductive loss, infertility, stillbirths, congenital anomalies, abnormal sexual development, mental retardation and pathogenesis of malignancy [1]. Clinical features of patients with common autosomal or sex chromosome aneuploidy is shown in Table 1. © 2015 The Author(s). Licensee InTech. This chapter is distributed under the terms of the Creative Commons Attribution License (http://creativecommons.org/licenses/by/3.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited. -

Original Article
Original article AN UNUSUAL CASE OF DOUBLE ANEUPLOIDY OF DOWN SYNDROME ASSOCIATED WITH TRIPLE X SYNDROME: 48,XXX,+21 A.Uwineza1, J.Hitayezu1, S.Murorunkwere1, J.Ndinkabandi1, L.Mutesa1,* 1Center for Medical Genetics, Faculty of Medicine, National University of Rwanda, Butare, Rwanda. ABSTRACT Down syndrome is the most common chromosomal abnormality in humans with an estimated incidence of one case in 770 live births. However, the occurrence of double aneuploidy involving autosome and or sex chromosome is a very rare phenomenon in lives born and the majority of reported cases are presented in form of spontaneous abortions. Here, we are reporting a case of a Rwandan patient with combination of trisomy 21 and triple X syndrome. The proband was 8-month-old female with typical features of Down syndrome. In additional to Down syndrome features, the child presented with minor features of triple X syndrome characterized by hypotonia and seizures. Keywords: Double aneuploidy - Down syndrome - Triple X syndrome - 48, XXX,+21 RESUME La trisomie 21 ou syndrome de Down est la plus fréquente anomalie chromosomique chez l’homme avec une incidence estimée à 770 cas de nouveau- nés vivants dans le monde. Cependant, la survenue d’une double anomalie chromosomique aneuploide intéressant les chromosomes autosomes ou chromosomes sexuels est un événement très rare et la majorité des cas rapportés sont liés à des formes de fausses couches spontanées. Nous rapportons ici un cas d’un patient Rwandais portant une double aneuploidie de trisomie 21 et le syndrome de Triple X; (48,XXX,+21). Le proband était un sujet de sexe féminin âgé de 8 mois présentant des symptômes typiques du syndrome de Down. -

The Origin and Impact of Embryonic Aneuploidy
Hum Genet DOI 10.1007/s00439-013-1309-0 ORIGINAL INVESTIGATION The origin and impact of embryonic aneuploidy Elpida Fragouli • Samer Alfarawati • Katharina Spath • Souraya Jaroudi • Jonas Sarasa • Maria Enciso • Dagan Wells Received: 15 March 2013 / Accepted: 11 April 2013 Ó Springer-Verlag Berlin Heidelberg 2013 Abstract Despite the clinical importance of aneuploidy, divisions. All abnormalities appeared to be tolerated until surprisingly little is known concerning its impact during activation of the embryonic genome, after which some the earliest stages of human development. This study aimed forms started to decline in frequency. However, many an- to shed light on the genesis, progression, and survival of euploidies continued to have little impact, with affected different types of chromosome anomaly from the fertilized embryos successfully reaching the blastocyst stage. Results oocyte through the final stage of preimplantation devel- from the direct analyses of female meiotic divisions and opment (blastocyst). 2,204 oocytes and embryos were early embryonic stages suggest that chromosome errors examined using comprehensive cytogenetic methodology. present during preimplantation development have origins A diverse array of chromosome abnormalities was detec- that are more varied than those seen in later pregnancy, ted, including many forms never recorded later in devel- raising the intriguing possibility that the source of aneu- opment. Advancing female age was associated with ploidy might modulate impact on embryo viability. The dramatic increase in aneuploidy rate and complex chro- results of this study also narrow the window of time for mosomal abnormalities. Anaphase lag and congression selection against aneuploid embryos, indicating that most failure were found to be important malsegregation causing survive until the blastocyst stage and, since they are not mechanisms in oogenesis and during the first few mitotic detected in clinical pregnancies, must be lost around the time of implantation or shortly thereafter. -
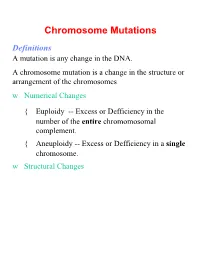
Chromosome Mutations Definitions a Mutation Is Any Change in the DNA
Chromosome Mutations Definitions A mutation is any change in the DNA. A chromosome mutation is a change in the structure or arrangement of the chromosomes w Numerical Changes { Euploidy -- Excess or Defficiency in the number of the entire chromomosomal complement. { Aneuploidy -- Excess or Defficiency in a single chromosome. w Structural Changes Numerical Chromosome Mutations Euploid -- “True Number” The cell(s) have the same number of copies of all the chromosome. 1. Monoploid (Haploid if a gamete) 2. Diploid (Often is not considered a Euploid) 3. Triploid 4. Tetraploid Euploidy Chromosomes shown at metaphase Normal Diploid Euploid Monoploid Triploid Tetraploid Euploidy is an excess or deficiency for the entire complement of chromosomes. Euploidy Organisms w Plants, amphibians, lower reptiles w Some cells in other organisms (liver, midgut, etc.) Root Causes w Nondisjunction w Autoreduplication Euploidy (continued) Origins w Autopolyploidy { Often involving individuals within the same species. { Infertile. Propagation by cloning. w Allopolyploidy { Often involving individuals from different species. { Fertile. Dosage Concerns Examples Numerical Chromosome Mutations Aneuploidy Excess or Defficiency in a single chromosome. 1. Monosomy 2. Disomy (only if normally cell is not diploid) 3. Trisomy 4. Tetrasomy Aneuploidy Chromosomes shown at metaphase Normal Diploid Aneuploid Monosomic Trisomic Tetrasomic Aneuploidy is an excess or deficiency for fewer than the entire complement of chromosomes. Aneuploidy Organisms w All organisms w Variability in how they deal with it Root Causes w Nondisjunction w Results of Chromosome Structure Mutations Aneuploidy -- Examples Plants w Jimson Weed (Datura stramonium) Animals w Drosophila: Triploid females are viable & fertile. Trisomy flies are not! w Human (autosomal): { Trisomy 13 (Patau Syndrome) 1:20,000 { Trisomy 18 (Edwards Syndrome) 1:10,000 { Trisomy 21 (Down Syndrome) 1:700 { Other rare ones (7, 9, 12, 14, & 22) Aneuploidy Examples w Human Sex Determining Chromosomes 1. -

50 Chromosomes
Leukemia (1998) 12, 427–433 1998 Stockton Press All rights reserved 0887-6924/98 $12.00 BIOTECHNICAL METHODS SECTION (BTS) BTS Leukemia Detection of hyperdiploid karyotypes (Ͼ50 chromosomes) in childhood acute lymphoblastic leukemia (ALL) using fluorescence in situ hybridization (FISH) J Ritterbach1, W Hiddemann2, JD Beck3, M Schrappe4, G Janka-Schaub5, W-D Ludwig6, J Harbott1 and F Lampert1 ¨ 1Oncogenetic Laboratory, Children’s Hospital, University of Gieen; 2Dept of Internal Medicine, University of Gottingen; 3Children’s Hospital, University of Erlangen; 4Children’s Hospital, Medical School of Hannover; 5Children’s Hospital, University of Hamburg; and ¨ 6Robert-Rossle-Clinic, Dept of Hematology, Oncology and Tumor Immunology, Humboldt-University, Berlin, Germany ALL patients with a hyperdiploid karyotype of more than 50 low white blood cell count and common or pre-B cell immu- chromosomes (high hyperdiploidy) carry a better prognosis in nophenotype.6 Moreover patients presenting with more than contrast to patients presenting with other cytogenetic features, and an appropriate less intensive therapy protocol should be 50 chromosomes in their blast cells have a favorable prog- developed for these patients. For this reason it is desirable to nosis compared to children belonging to other karyotypic sub- have a quick screening method identifying those with this type groups.7,8 Karyotyping of patients with ALL, however, is diffi- of hyperdiploidy. We therefore studied the bone marrow and/or cult because of the poor quality of metaphases. Flow blood cells of 278 children with ALL using double target fluor- cytometric measurements of the cellular DNA will identify the escence in situ hybridization (FISH) on interphase. -

Aneuploidy Vs. Gene Mutation Hypothesis of Cancer: Recent Study Claims Mutation but Is Found to Support Aneuploidy
Aneuploidy vs. gene mutation hypothesis of cancer: Recent study claims mutation but is found to support aneuploidy Ruhong Li, Arvind Sonik, Reinhard Stindl, David Rasnick, and Peter Duesberg* Department of Molecular and Cell Biology, Stanley Hall, University of California, Berkeley, CA 94720 Contributed by Peter H. Duesberg, December 6, 1999 For nearly a century, cancer has been blamed on somatic mutation. mutations; (vi) cancers are diploid, because gene mutations do But it is still unclear whether this mutation is aneuploidy, an not depend on karyotype alterations for expression. abnormal balance of chromosomes, or gene mutation. Despite However, these predictions have been difficult to meet. (i) enormous efforts, the currently popular gene mutation hypothesis There is a growing list of nongenotoxic carcinogens, including has failed to identify cancer-specific mutations with transforming asbestos, Ni2ϩ, hormones, butter yellow, arsenic, acrylamide, function and cannot explain why cancer occurs only many months urethan, etc. (13–17). (ii) No cancer-specific gene mutations to decades after mutation by carcinogens and why solid cancers are have been found yet (18–23). According to a recent commentary aneuploid, although conventional mutation does not depend on (‘‘How many mutations does it take to make a tumor?’’), karyotype alteration. A recent high-profile publication now claims ‘‘There are no oncogenes or tumor suppressor genes that are to have solved these discrepancies with a set of three synthetic activated or deleted from all cancers. Even tumors of a single mutant genes that ‘‘suffices to convert normal human cells into organ rarely have uniform genetic alterations, although tumor tumorigenic cells.’’ However, we show here that even this study types from one specific organ have a tendency to share muta- failed to explain why it took more than ‘‘60 population doublings’’ tions.’’ (24). -

XYY Syndrome (Jacobs Syndrome)
General information about positive NIPT results What testing could be considered? My patient’s NIPT is positive for XYY syndrome (Jacobs syndrome). What does this mean? Your patient’s NIPT result • Specialized genetic tests such as karyotyping, fluorescence in suggests the presence of an extra copy of the Y chromosome. situ hybridization (FISH), qPCR and microarray are available to NIPT is a screening test; false positives can occur. The actual confirm the presence of XYY syndrome. chance for the pregnancy to have XYY syndrome depends on • These confirmatory tests are generally performed on cells from many factor, including the patient’s clinical and family history. chorionic villus sampling (CVS) or amniocentesis during Next steps to consider: You should discuss the results pregnancy, on cord blood or peripheral blood sample after the and the potential clinical implications with your patient. The baby is born, or on products of conception (POC) in case of American College of Obstetricians and Gynecologists and the a miscarriage. Society for Maternal-Fetal Medicine state, “All women with a • Ultrasound evaluation is not expected to be useful in aiding with positive cell-free DNA test result should have further detailed a prenatal diagnosis of XYY syndrome, as ultrasound is usually counseling and testing and should have a diagnostic procedure normal. A normal ultrasound cannot exclude this condition. before any irreversible action is taken.”1 Confirmation prior to birth can also help with pregnancy and neonatal management. Resources for