In Potato Plant 126, 127
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Functional Genomics of Phytophthora Infestans Effectors and Solanum Resistance Genes
Functional Genomics of Phytophthora infestans Effectors and Solanum Resistance Genes Nicolas Champouret Thesis committee Thesis supervisors Prof. dr. Richard G.F. Visser Professor of Plant Breeding Wageningen University Prof. dr. Evert Jacobsen Professor of Plant Breeding Wageningen University Thesis co-supervisor Dr. Vivianne G.A.A. Vleeshouwers Researcher Wageningen University Other members Prof. Dr. Ir. Pierre J. G. M. de Wit, Wageningen University, The Netherlands Prof. Dr. Martien Groenen, Wageningen University, The Netherlands Prof. Dr. Ir. Corné Pieterse, Utrecht University, The Netherlands Dr. Brande Wulff, The Sainsbury Laboratory, Norwich, UK This research was conducted under the auspices of the Graduate School of Experimental Plant Sciences. II Functional Genomics of Phytophthora infestans Effectors and Solanum Resistance Genes Nicolas Champouret Thesis submitted in partial fulfillment of the requirements for the degree of doctor at Wageningen University by the authority of the Rector Magnificus Prof. dr. M.J. Kropff in the presence of the Thesis Committee appointed by the Doctorate Board to be defended in public on Wednesday 9 June 2010 at 4 p.m. in the Aula. III Nicolas Champouret Functional Genomics of Phytophthora infestans Effectors and Solanum Resistance Genes. 162 pages Thesis, Wageningen University, Wageningen, NL (2010) With references, with summaries in Dutch and English ISBN 978-90-8585-658-0 IV CONTENTS Abstract VII Chapter 1 1 General introduction Chapter 2 15 Phytophthora infestans Isolates Lacking Class I ipiO Variants Are Virulent on Rpi-blb1 Potato Chapter 3 43 Evolutionary and Functional Analyses Reveal a Diverse Family of R2 Late Blight Resistance Genes in Mexican Solanum Species Chapter 4 75 Diversity of PiAvr2/PexRD11 and R2 gene families underpins co-evolution between Phytophthora infestans and Mexican Solanum species Chapter 5 90 Functional allele-mining with Avr3a reveals active R3a in S. -

How Many Fungi Make Sclerotia?
fungal ecology xxx (2014) 1e10 available at www.sciencedirect.com ScienceDirect journal homepage: www.elsevier.com/locate/funeco Short Communication How many fungi make sclerotia? Matthew E. SMITHa,*, Terry W. HENKELb, Jeffrey A. ROLLINSa aUniversity of Florida, Department of Plant Pathology, Gainesville, FL 32611-0680, USA bHumboldt State University of Florida, Department of Biological Sciences, Arcata, CA 95521, USA article info abstract Article history: Most fungi produce some type of durable microscopic structure such as a spore that is Received 25 April 2014 important for dispersal and/or survival under adverse conditions, but many species also Revision received 23 July 2014 produce dense aggregations of tissue called sclerotia. These structures help fungi to survive Accepted 28 July 2014 challenging conditions such as freezing, desiccation, microbial attack, or the absence of a Available online - host. During studies of hypogeous fungi we encountered morphologically distinct sclerotia Corresponding editor: in nature that were not linked with a known fungus. These observations suggested that Dr. Jean Lodge many unrelated fungi with diverse trophic modes may form sclerotia, but that these structures have been overlooked. To identify the phylogenetic affiliations and trophic Keywords: modes of sclerotium-forming fungi, we conducted a literature review and sequenced DNA Chemical defense from fresh sclerotium collections. We found that sclerotium-forming fungi are ecologically Ectomycorrhizal diverse and phylogenetically dispersed among 85 genera in 20 orders of Dikarya, suggesting Plant pathogens that the ability to form sclerotia probably evolved 14 different times in fungi. Saprotrophic ª 2014 Elsevier Ltd and The British Mycological Society. All rights reserved. Sclerotium Fungi are among the most diverse lineages of eukaryotes with features such as a hyphal thallus, non-flagellated cells, and an estimated 5.1 million species (Blackwell, 2011). -

Pest Management Strategic Plan for Organic Potato Production in the West
Pest Management Strategic Plan for Organic Potato Production in the West Summary of workshops held on February 16, 2006 Buhl, Idaho and January 9, 2008 Portland, Oregon Issue Date December 19, 2008 Lead Authors: Jennifer Miller, Ronda Hirnyck, Lisa Downey-Blecker Editor: Diane Clarke This project was sponsored by the Western Integrated Pest Management Center, which is funded by the United States Department of Agriculture, Cooperative State Research, Education, and Extension Service. Additional funding was provided by the Organic Farming Research Foundation and the Bullitt Foundation. Table of Contents Work Group .........................................................................................................................3 Summary of the Most Critical Needs in Organic Potato Production in the West ................5 Introduction ..........................................................................................................................6 Production Overview .........................................................................................................11 California ...............................................................................................................11 Colorado .................................................................................................................12 Columbia Basin ......................................................................................................12 Idaho ......................................................................................................................13 -

Mycologist News
MYCOLOGIST NEWS The newsletter of the British Mycological Society 2012 (4) Edited by Prof. Pieter van West and Dr Anpu Varghese 2013 BMS Council BMS Council and Committee Members 2013 President Prof. Geoffrey D. Robson Vice-President Prof. Bruce Ing President Elect Prof Nick Read Treasurer Prof. Geoff M Gadd Secretary Position vacant Publications Officer Dr. Pieter van West International Initiatives Adviser Prof. AJ Whalley Fungal Biology Research Committee representatives: Dr. Elaine Bignell; Prof Nick Read Fungal Education and Outreach Committee: Dr. Paul S. Dyer; Dr Ali Ashby Field Mycology and Conservation: Dr. Stuart Skeates, Mrs Dinah Griffin Fungal Biology Research Committee Prof. Nick Read (Chair) retiring 31.12. 2013 Dr. Elaine Bignell retiring 31.12. 2013 Dr. Mark Ramsdale retiring 31.12. 2013 Dr. Pieter van West retiring 31.12. 2013 Dr. Sue Crosthwaite retiring 31.12. 2014 Prof. Mick Tuite retiring 31.12. 2014 Dr Alex Brand retiring 31.12. 2015 Fungal Education and Outreach Committee Dr. Paul S. Dyer (Chair and FBR link) retiring 31.12. 2013 Dr. Ali Ashby retiring 31.12. 2013 Ms. Carol Hobart (FMC link) retiring 31.12. 2012 Dr. Sue Assinder retiring 31.12. 2013 Dr. Kay Yeoman retiring 31.12. 2013 Alan Williams retiring 31.12. 2014 Prof Lynne Boddy (Media Liaison) retiring 31.12. 2014 Dr. Elaine Bignell retiring 31.12. 2015 Field Mycology and Conservation Committee Dr. Stuart Skeates (Chair, website & FBR link) retiring 31.12. 2014 Prof Richard Fortey retiring 31.12. 2013 Mrs. Sheila Spence retiring 31.12. 2013 Mrs Dinah Griffin retiring 31.12. 2014 Dr. -

PCN Guidelines, and Potato Cyst Nematodes (Globodera Rostochiensis Or Globodera Pallida) Were Not Detected.”
Canada and United States Guidelines on Surveillance and Phytosanitary Actions for the Potato Cyst Nematodes Globodera rostochiensis and Globodera pallida 7 May 2014 Table of Contents 1. Introduction ...........................................................................................................................................................3 2. Rationale for phytosanitary actions ........................................................................................................................3 3. Soil sampling and laboratory analysis procedures .................................................................................................4 4. Phytosanitary measures ........................................................................................................................................4 5. Regulated articles .................................................................................................................................................5 6. National PCN detection survey..............................................................................................................................6 7. Pest-free places of production or pest-free production sites within regulated areas ...............................................6 8. Phytosanitary certification of seed potatoes ..........................................................................................................7 9. Releasing land from regulatory control ..................................................................................................................8 -

The Fungi of Slapton Ley National Nature Reserve and Environs
THE FUNGI OF SLAPTON LEY NATIONAL NATURE RESERVE AND ENVIRONS APRIL 2019 Image © Visit South Devon ASCOMYCOTA Order Family Name Abrothallales Abrothallaceae Abrothallus microspermus CY (IMI 164972 p.p., 296950), DM (IMI 279667, 279668, 362458), N4 (IMI 251260), Wood (IMI 400386), on thalli of Parmelia caperata and P. perlata. Mainly as the anamorph <it Abrothallus parmeliarum C, CY (IMI 164972), DM (IMI 159809, 159865), F1 (IMI 159892), 2, G2, H, I1 (IMI 188770), J2, N4 (IMI 166730), SV, on thalli of Parmelia carporrhizans, P Abrothallus parmotrematis DM, on Parmelia perlata, 1990, D.L. Hawksworth (IMI 400397, as Vouauxiomyces sp.) Abrothallus suecicus DM (IMI 194098); on apothecia of Ramalina fustigiata with st. conid. Phoma ranalinae Nordin; rare. (L2) Abrothallus usneae (as A. parmeliarum p.p.; L2) Acarosporales Acarosporaceae Acarospora fuscata H, on siliceous slabs (L1); CH, 1996, T. Chester. Polysporina simplex CH, 1996, T. Chester. Sarcogyne regularis CH, 1996, T. Chester; N4, on concrete posts; very rare (L1). Trimmatothelopsis B (IMI 152818), on granite memorial (L1) [EXTINCT] smaragdula Acrospermales Acrospermaceae Acrospermum compressum DM (IMI 194111), I1, S (IMI 18286a), on dead Urtica stems (L2); CY, on Urtica dioica stem, 1995, JLT. Acrospermum graminum I1, on Phragmites debris, 1990, M. Marsden (K). Amphisphaeriales Amphisphaeriaceae Beltraniella pirozynskii D1 (IMI 362071a), on Quercus ilex. Ceratosporium fuscescens I1 (IMI 188771c); J1 (IMI 362085), on dead Ulex stems. (L2) Ceriophora palustris F2 (IMI 186857); on dead Carex puniculata leaves. (L2) Lepteutypa cupressi SV (IMI 184280); on dying Thuja leaves. (L2) Monographella cucumerina (IMI 362759), on Myriophyllum spicatum; DM (IMI 192452); isol. ex vole dung. (L2); (IMI 360147, 360148, 361543, 361544, 361546). -
Weed ID Guide
GUIDE Cross Sector Weed ID guide 1 Contents Introduction How best to use this pocket guide 2 Weeds as alternative hosts to pests and diseases 3 Importance of weed species in each sector 3 Non-chemical weed control 3 Key features of weeds Key features of weeds to aid identification 4 Broad-leaved weed seedlings 4 Grass weed seedlings 6 Identification groups key Identification groups 1–26 8 Importance of each weed Table 1. Relative importance of each weed in each horticultural sector i Non-chemical weed control Table 2. Ease of non-chemical weed control iv Additional Information Acknowledgements viii 0.A 2 Introduction A pocket guide to aid the identification of the most widespread and economically important weeds encountered in the horticultural sector, this publication covers mostly broad-leaved weeds but also some grasses, moss and liverwort. How best to use this pocket guide This guide is primarily an aid to weed seedling identification to help growers choose the right cultural or herbicidal control method. To correctly identify a weed seedling, first read the section, ‘Key features of weeds’, as this will provide a structured plan of how to approach the identification process. Familiarisation with the various parts of the seedling and checking the key features is essential for correct identification. The weeds are grouped (1–26), by common features of the seedlings, so that this facilitates quicker identification. Each weed has been photographed at three stages of its development: cotyledon, first/second true leaf and mature plant, to allow it to be identified at all stages of growth. -

Testing Taxonomic Predictivity of Foliar and Tuber Resistance to Phytophthora Infestans in Wild Relatives of Potato
Genetics and Resistance Testing Taxonomic Predictivity of Foliar and Tuber Resistance to Phytophthora infestans in Wild Relatives of Potato A. Khiutti, D. M. Spooner, S. H. Jansky, and D. A. Halterman First author: All-Russian Institute for Plant Protection, Laboratory of Plant Immunity to Diseases, 3, Podbelsky shosse, St. Petersburg-Pushkin, 196608, Russia; second, third, and fourth authors: United States Department of Agriculture–Agricultural Research Service, Madison, WI, 53726; and second and third authors: Department of Horticulture, University of Wisconsin, Madison 53706. Accepted for publication 8 April 2015. ABSTRACT Khiutti, A., Spooner, D. M., Jansky, S. H., and Halterman, D. A. 2015. intensive. We tested the ability of taxonomy, ploidy, crossing group, Testing taxonomic predictivity of foliar and tuber resistance to Phytoph- breeding system, and geography to predict the presence of foliar and thora infestans in wild relatives of potato. Phytopathology 105:1198-1205. tuber late blight resistance in wild Solanum spp. Significant variation for resistance to both tuber and foliar late blight was found within and Potato late blight, caused by the oomycete phytopathogen Phytoph- among species but there was no discernable predictive power based on thora infestans, is a devastating disease found in potato-growing regions taxonomic series, clade, ploidy, breeding system, elevation, or geo- worldwide. Long-term management strategies to control late blight graphic location. We observed a moderate but significant correlation include the incorporation of host resistance to predominant strains. between tuber and foliar resistance within species. Although previously However, due to rapid genetic changes within pathogen populations, uncharacterized sources of both foliar and tuber resistance were rapid and recurring identification and integration of novel host resistance identified, our study does not support an assumption that taxonomic or traits is necessary. -

Potato - Wikipedia, the Free Encyclopedia
Potato - Wikipedia, the free encyclopedia Log in / create account Article Talk Read View source View history Our updated Terms of Use will become effective on May 25, 2012. Find out more. Main page Potato Contents From Wikipedia, the free encyclopedia Featured content Current events "Irish potato" redirects here. For the confectionery, see Irish potato candy. Random article For other uses, see Potato (disambiguation). Donate to Wikipedia The potato is a starchy, tuberous crop from the perennial Solanum tuberosum Interaction of the Solanaceae family (also known as the nightshades). The word potato may Potato Help refer to the plant itself as well as the edible tuber. In the region of the Andes, About Wikipedia there are some other closely related cultivated potato species. Potatoes were Community portal first introduced outside the Andes region four centuries ago, and have become Recent changes an integral part of much of the world's cuisine. It is the world's fourth-largest Contact Wikipedia food crop, following rice, wheat and maize.[1] Long-term storage of potatoes Toolbox requires specialised care in cold warehouses.[2] Print/export Wild potato species occur throughout the Americas, from the United States to [3] Uruguay. The potato was originally believed to have been domesticated Potato cultivars appear in a huge variety of [4] Languages independently in multiple locations, but later genetic testing of the wide variety colors, shapes, and sizes Afrikaans of cultivars and wild species proved a single origin for potatoes in the area -
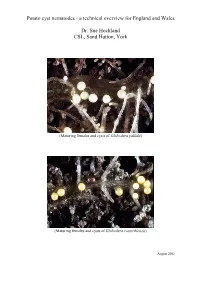
Potato Cyst Nematodes - a Technical Overview for England and Wales
Potato cyst nematodes - a technical overview for England and Wales Dr. Sue Hockland CSL, Sand Hutton, York (Maturing females and cysts of Globodera pallida) (Maturing females and cysts of Globodera rostochiensis) August 2002 Contents Page • Executive Summary 1 • Introduction 2 • PCN: species and diagnosis 2 • Biology of PCN species 3 • Detection 4 • Host plants of PCN 4 • Symptoms 5 • Damage 5 • Pathotypes and Host Plant Resistance 5 • Distribution and spread in England and Wales 6 • Distribution in Europe and elsewhere 9 • Statutory Management of PCN 11 • Management of PCN in ware potatoes 13 • Chemical methods 14 • Non-chemical methods 14 • Conclusion 16 • Acknowledgements 17 Executive Summary The pests Potato cyst nematode (PCN) is the name commonly given to two species of cyst nematode that attack potato, namely Globodera pallida (Stone) Behrens and G. rostochiensis (Wollenweber) Behrens. They are two of the most important pests of potato in England and Wales, feeding on potato roots, causing losses of yield and costs that vary and are difficult to estimate. Published papers usually quote losses of about 9% of annual yield, estimated at about £43 million for the UK, based on the mean value of the crop from 1990-1995. Adaptations to a plant-parasitic life In both species the female forms a hard covering around her eggs when she dies, creating a ‘cyst’ which protects the eggs and developing juveniles from desiccation, predation and chemical control. Only a proportion of the eggs hatch from the cyst each year, and in G. pallida this occurs at a slower rate and with a later annual peak of hatching than G. -

Open Ohlsondissertation.Pdf
The Pennsylvania State University The Graduate School Intercollege Graduate Degree Program in Genetics GENETIC CHARACTERIZATION AND MAPPING OF LATE BLIGHT RESISTANCE GENES IN THE WILD TOMATO ACCESSIONS PI 163245 AND PI 224710 A Dissertation in Genetics by Erik William Ohlson © 2015 Erik William Ohlson Submitted in Partial Fulfillment of the Requirements for the Degree of Doctor of Philosophy December 2015 ii The dissertation of Erik William Ohlson was reviewed and approved* by the following: Majid R. Foolad Professor of Plant Genetics Dissertation Advisor David R. Huff Professor of Turfgrass Breeding and Genetics Chair of Committee Surinder Chopra Professor of Maize Genetics Beth K. Gugino Associate Professor of Vegetable Pathology Timothy W. McNellis Associate Professor of Plant Pathology Yinong Yang Associate Professor of Plant Pathology Robert F. Paulson Professor of Veterinary and Biomedical Sciences Chair of the Intercollege Graduate Degree Program in Genetics *Signatures are on file in the Graduate School. iii ABSTRACT Late blight (LB), caused by the oomycete Phytophthora infestans (Mont.) de Bary is one of the most destructive diseases of tomato and potato worldwide. Development of fungicide resistant and more aggressive P. infestans clonal lineages has emphasized the importance of discovering and incorporating new genetic resistance in tomato cultivars. Although the cultivated tomato, Solanum lycopersicum L., contains limited genetic diversity, several related wild species of tomato are suitable for identification of new desirable traits. Previously, 67 S. pimpinellifolium accessions were screened for LB resistance in field, greenhouse and detached leaflet trials and 12 accessions with strong resistance to LB were identified. In this dissertation, two resistant accessions, PI 163245 and PI 224710, were selected for further genetic characterization. -

Gene RB Cloned from Solanum Bulbocastanum Confers Broad Spectrum Resistance to Potato Late Blight
Gene RB cloned from Solanum bulbocastanum confers broad spectrum resistance to potato late blight Junqi Song*†, James M. Bradeen†‡, S. Kristine Naess‡, John A. Raasch§, Susan M. Wielgus*‡, Geraldine T. Haberlach‡, Jia Liu¶, Hanhui Kuangʈ, Sandra Austin-Phillips§, C. Robin Buell¶, John P. Helgeson‡**, and Jiming Jiang*,** *Department of Horticulture, §Biotechnology Center, and ‡U.S. Department of Agriculture–Agricultural Research Service and Department of Plant Pathology, University of Wisconsin, Madison, WI 53706; ¶The Institute for Genomic Research, 9712 Medical Center Drive, Rockville, MD 20850; and ʈDepartment of Vegetable Crops, University of California, Davis, CA 95616 Communicated by S. J. Peloquin, University of Wisconsin, Madison, WI, June 6, 2003 (received for review March 1, 2003) Late blight, caused by the oomycete pathogen Phytophthora times sporulates on PT29-derived resistant materials. The resis- infestans, is the most devastating potato disease in the world. tance of the PT29-derived plants is manifested as a slow Control of late blight in the United States and other developed progression of lesion development that substantially decreases countries relies extensively on fungicide application. We previ- the rate of disease development in the plants. This phenotype of ously demonstrated that the wild diploid potato species Solanum general suppression but not elimination of symptom develop- bulbocastanum is highly resistant to all known races of P. infestans. ment has been consistently observed in field tests at various Potato germplasm derived from S. bulbocastanum has shown locations in the United States and in Toluca, Mexico, between durable and effective resistance in the field. Here we report the 1995 and 2002. The late blight resistance associated with the cloning of the major resistance gene RB in S.