National Essential Medicine List of Afghanistan
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-
Extended-Release 30 Mcg Capsules Service Request Form Fax: 1-844-660-7083 | Phone: 1-844-414-Opko (6756) E-Mail: [email protected]
RAYALDEE® (CALCIFEDIOL) EXTENDED-RELEASE 30 MCG CAPSULES SERVICE REQUEST FORM FAX: 1-844-660-7083 | PHONE: 1-844-414-OPKO (6756) E-MAIL: [email protected] 1. Patient Information Please complete all fields to prevent any delays. New start to Rayaldee® therapy Existing patient on therapy E-mail Address: Preferred Method of Contact: Cell Phone Home Phone Email Text First Name Last Name SS # (Last 4 only) Preferred Time of Contact: Morning Afternoon Evening Male Female Ok to leave a message: Yes No Date of Birth (MM/DD/YYYY) Primary Language: English Spanish Other:________________________________ 2. Patient Insurance Information Address Attached is a copy of both sides of the patient's insurance card City State ZIP Primary Insurance Phone # Cell Phone Home Phone Policy Holder Name Relationship to Patient Alternate Contact or Healthcare Proxy First Name, Last Name and Phone Insurance ID # Group # 3. Patient Clinical Information Please include supporting clinical documentation. ● ICD-10 Code: ● Lab Values & dates: Please check box 1. or 2. below 25(OH)D__________|__________ Calcium__________|__________ (N18.3) CKD stage 3, (N25.81) Secondary hyperparathyroidism, and 1. value date value date (E55.9) Vitamin D deficiency 2. (N18.4) CKD stage 4, (N25.81) Secondary hyperparathyroidism, and (E55.9) Vitamin D deficiency ● Therapies within the previous 6 months: No previous therapies Hectorol® (doxercalciferol) Rocaltrol® (calcitriol) OTC Vitamin D2 ® OTC Vitamin D3 Prescription Vitamin D2 (ergocalciferol) Zemplar (paricalcitol) 4. Prescriber Information Specialty of Prescriber: Nephrologist PCP Endocrinologist Internist First Name Last Name Phone Fax Practice Name Oce Contact Preferred Time of Contact Address NPI # City State ZIP 5. -
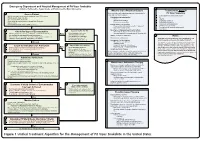
Snake Bite Protocol
Lavonas et al. BMC Emergency Medicine 2011, 11:2 Page 4 of 15 http://www.biomedcentral.com/1471-227X/11/2 and other Rocky Mountain Poison and Drug Center treatment of patients bitten by coral snakes (family Ela- staff. The antivenom manufacturer provided funding pidae), nor by snakes that are not indigenous to the US. support. Sponsor representatives were not present dur- At the time this algorithm was developed, the only ing the webinar or panel discussions. Sponsor represen- antivenom commercially available for the treatment of tatives reviewed the final manuscript before publication pit viper envenomation in the US is Crotalidae Polyva- ® for the sole purpose of identifying proprietary informa- lent Immune Fab (ovine) (CroFab , Protherics, Nash- tion. No modifications of the manuscript were requested ville, TN). All treatment recommendations and dosing by the manufacturer. apply to this antivenom. This algorithm does not con- sider treatment with whole IgG antivenom (Antivenin Results (Crotalidae) Polyvalent, equine origin (Wyeth-Ayerst, Final unified treatment algorithm Marietta, Pennsylvania, USA)), because production of The unified treatment algorithm is shown in Figure 1. that antivenom has been discontinued and all extant The final version was endorsed unanimously. Specific lots have expired. This antivenom also does not consider considerations endorsed by the panelists are as follows: treatment with other antivenom products under devel- opment. Because the panel members are all hospital- Role of the unified treatment algorithm -

Anthem Blue Cross Drug Formulary
Erythromycin/Sulfisoxazole (generic) INTRODUCTION Penicillins ...................................................................... Anthem Blue Cross uses a formulary Amoxicillin (generic) (preferred list of drugs) to help your doctor Amoxicillin/Clavulanate (generic/Augmentin make prescribing decisions. This list of drugs chew/XR) is updated quarterly, by a committee Ampicillin (generic) consisting of doctors and pharmacists, so that Dicloxacillin (generic) the list includes drugs that are safe and Penicillin (generic) effective in the treatment of diseases. If you Quinolones ..................................................................... have any questions about the accessibility of Ciprofloxacin/XR (generic) your medication, please call the phone number Levofloxacin (Levaquin) listed on the back of your Anthem Blue Cross Sulfonamides ................................................................ member identification card. Erythromycin/Sulfisoxazole (generic) In most cases, if your physician has Sulfamethoxazole/Trimethoprim (generic) determined that it is medically necessary for Sulfisoxazole (generic) you to receive a brand name drug or a drug Tetracyclines .................................................................. that is not on our list, your physician may Doxycycline hyclate (generic) indicate “Dispense as Written” or “Do Not Minocycline (generic) Substitute” on your prescription to ensure Tetracycline (generic) access to the medication through our network ANTIFUNGAL AGENTS (ORAL) _________________ of community -

Poisoning – Investigative and Management Protocols
UNIT-1 Management of Common Clinical Poisoning Dr. Pallav Shekhar Asstt. Professor Veterinary Medicine Suspection Common Symptoms exhibited by large no. of animals at a time. ➢ Sudden death ➢ Salivation ➢ Vomition ➢ Neurological signs ➢ Presence of mouldy feeds ➢ Presence of house hold waste and medicaments ➢ Sewage water contamination ➢ Presence of dead rat or rat wait ➢ Spray of insecticides, weedicides. ➢ Use of paint Principal of treatment Prevention of further exposure Alkalis are more dangerous than acids. Acids form insoluble acid proteinates and hence its effect is partially self limiting Alkalis form alkali proteinates along with soaps which will penetrate rapidly into tissues. Inhaled poisons can be eliminated by providing assisted ventilation. Topical applied toxicant can be removed by washing with plenty of water and soap. Skin contact can be eliminated by washing with plenty of water and soap if the poison is water soluble and with organic solvents like Benzene, alcohol if they are fat soluble Conti…. Clipping of hair or wool may be necessary Emesis is of value in dogs, cats and pigs if done within few hours of ingestion. Emesis is contraindicated when ▪ The swallowing reflex is absent ▪ Animal is convulsive ▪ Corrosive agent or volatile hydrocarbons or petroleum product ingested Conti…. Emetics: a) Oral: I. Syrup of ipecac; 10-20ml in dogs II. Hydrogen peroxide: 2ml/kg b) Parenteral: Apomorphine can be used in dogs at dosage of 0.05-0.1mg/kg Conti… Gastric lavage: This is done in mono-gastric animals and 10ml lavage fluid/kg body weight should be given. Potassium permanganate solution This is done with Endotracheal tube and stomach tube Conti… Insertion of stomach tube in cattle for gastric lavage Conti. -

Potassium Iodide (KI): Instructions for Children
Potassium Iodide (KI): Instructions for Children The thyroid gland in children is very sensitive to the effects of radioactive iodine. In the event of a nuclear emergency, it is important for adults to understand how to prepare the proper dosage of potassium iodide (KI) for young children. The following information will help you to give KI to your children properly. Children over 12 years to 18 years 2 tablets (whole or crushed) (130 mg) (who weigh at least 150 pounds) Children over 12 years to 18 years 1 tablet (whole or crushed) or 8 teaspoons (65 mg) (who weigh less than 150 pounds) Children over 3 years to 12 years 1 tablet (whole or crushed) or 8 teaspoons (65 mg) Children over 1 month to 3 years 4 teaspoons (32.5 mg) Babies at birth to 1 month 2 teaspoons (16.25 mg) Tablets can be crushed and mixed in many liquids. To take the tablet in liquid solution, use dosing directions under “Making a Potassium Iodide Liquid Mixture.” Take KI only as directed by public officials. Do not take more than 1 dose in 24 hours. More will not help you. Too much medicine may increase the chances of side effects. Making a Potassium Iodide Liquid Mixture 1. Put one 65 mg KI tablet into a small bowl and grind it into a fine powder using the back of a metal teaspoon against the inside of the bowl. The powder should not have any large pieces. 2. Add 4 teaspoons of water to the crushed KI powder in the bowl and mix until the KI powder is dissolved in the water. -

Determination of Iodate in Iodised Salt by Redox Titration
College of Science Determination of Iodate in Iodised Salt by Redox Titration Safety • 0.6 M potassium iodide solution (10 g solid KI made up to 100 mL with distilled water) • 0.5% starch indicator solution Lab coats, safety glasses and enclosed footwear must (see below for preparation) be worn at all times in the laboratory. • 250 mL volumetric flask Introduction • 50 mL pipette (or 20 and 10 mL pipettes) • 250 mL conical flasks New Zealand soil is low in iodine and hence New Zealand food is low in iodine. Until iodised salt was • 10 mL measuring cylinder commonly used (starting in 1924), a large proportion • burette and stand of school children were reported as being affected • distilled water by iodine deficiency – as high as 60% in Canterbury schools, and averaging 20 − 40% overall. In the worst cases this deficiency can lead to disorders such as Method goitre, and impaired physical and mental development. 1. Preparation of 0.002 mol L−1 sodium thiosulfate In earlier times salt was “iodised” by the addition of solution: Accurately weigh about 2.5 g of solid potassium iodide; however, nowadays iodine is more sodium thiosulfate (NaS2O3•5H2O) and dissolve in commonly added in the form of potassium iodate 100 mL of distilled water in a volumetric flask. (This gives a 0.1 mol L−1 solution). Then use a pipette to (KIO3). The Australia New Zealand Food Standards Code specifies that iodised salt must contain: “equivalent to transfer 10 mL of this solution to a 500 mL volumetric no less than 25 mg/kg of iodine; and no more than 65 flask and dilute by adding distilled water up to the mg/kg of iodine”. -

Radionuclide Thyroid Scans
Radionuclide Thyroid Scans Report 2003 1 Purpose The purpose of this guideline is to assist specialists in Nuclear Medicine and Radionuclide Radiology in recommending, performing, interpreting and reporting radionuclide thyroid scans. This guideline will assist individual departments in the formulation of their own local protocols. Background Thyroid scintigraphy is an effective imaging method for assessing the functionality of thyroid lesions including the uptake function of part or all of the thyroid gland. 99TCm pertechnetate is trapped by thyroid follicular cells. 123I-Iodide is both trapped and organified by thyroid follicular cells. Common Indications 1.1 Assessment of functionality of thyroid nodules. 1.2 Assessment of goitre including hyperthyroid goitre. 1.3 Assessment of uptake function prior to radio-iodine treatment 1.4 Assessment of ectopic thyroid tissue. 1.5 Assessment of suspected thyroiditis 1.6 Assessment of neonatal hypothyroidism Procedure 1 Patient preparation 1.1 Information on patient medication should be obtained prior to undertaking study. Patients on Thyroxine (Levothyroxine Sodium) should stop treatment for four weeks prior to imaging, patients on Tri-iodothyronine (T3) should stop treatment for two weeks if adequate images are to be obtained. 1.2 All relevant clinical history should be obtained on attendance, including thyroid medication, investigations with contrast media, other relevant medication including Amiodarone, Lithium, kelp, previous surgery and diet. 1.3 All other relevant investigations should be available including results of thyroid function tests and ultrasound examinations. 1.4 Studies should be scheduled to avoid iodine-containing contrast media prior to thyroid imaging. 2 1.5 Carbimazole and Propylthiouracil are not contraindicated in patients undergoing 99Tcm pertechnetate thyroid scans and need not be discontinued prior to imaging. -

)&F1y3x PHARMACEUTICAL APPENDIX to THE
)&f1y3X PHARMACEUTICAL APPENDIX TO THE HARMONIZED TARIFF SCHEDULE )&f1y3X PHARMACEUTICAL APPENDIX TO THE TARIFF SCHEDULE 3 Table 1. This table enumerates products described by International Non-proprietary Names (INN) which shall be entered free of duty under general note 13 to the tariff schedule. The Chemical Abstracts Service (CAS) registry numbers also set forth in this table are included to assist in the identification of the products concerned. For purposes of the tariff schedule, any references to a product enumerated in this table includes such product by whatever name known. Product CAS No. Product CAS No. ABAMECTIN 65195-55-3 ACTODIGIN 36983-69-4 ABANOQUIL 90402-40-7 ADAFENOXATE 82168-26-1 ABCIXIMAB 143653-53-6 ADAMEXINE 54785-02-3 ABECARNIL 111841-85-1 ADAPALENE 106685-40-9 ABITESARTAN 137882-98-5 ADAPROLOL 101479-70-3 ABLUKAST 96566-25-5 ADATANSERIN 127266-56-2 ABUNIDAZOLE 91017-58-2 ADEFOVIR 106941-25-7 ACADESINE 2627-69-2 ADELMIDROL 1675-66-7 ACAMPROSATE 77337-76-9 ADEMETIONINE 17176-17-9 ACAPRAZINE 55485-20-6 ADENOSINE PHOSPHATE 61-19-8 ACARBOSE 56180-94-0 ADIBENDAN 100510-33-6 ACEBROCHOL 514-50-1 ADICILLIN 525-94-0 ACEBURIC ACID 26976-72-7 ADIMOLOL 78459-19-5 ACEBUTOLOL 37517-30-9 ADINAZOLAM 37115-32-5 ACECAINIDE 32795-44-1 ADIPHENINE 64-95-9 ACECARBROMAL 77-66-7 ADIPIODONE 606-17-7 ACECLIDINE 827-61-2 ADITEREN 56066-19-4 ACECLOFENAC 89796-99-6 ADITOPRIM 56066-63-8 ACEDAPSONE 77-46-3 ADOSOPINE 88124-26-9 ACEDIASULFONE SODIUM 127-60-6 ADOZELESIN 110314-48-2 ACEDOBEN 556-08-1 ADRAFINIL 63547-13-7 ACEFLURANOL 80595-73-9 ADRENALONE -

Evaluating and Managing Patients with Thyrotoxicosis
Thyroid Evaluating and Kirsten Campbell managing patients with Matthew Doogue thyrotoxicosis Background Thyrotoxicosis is common in the Australian population Thyrotoxicosis is common in the Australian community and and thus a frequent clinical scenario facing the general is frequently encountered in general practice. Graves disease, practitioner. The prevalence of thyrotoxicosis (subclinical toxic multinodular goitre, toxic adenoma and thyroiditis or overt) reported among those without a history of thyroid account for most presentations of thyrotoxicosis. disease in Australia is approximately 0.5% and this Objective increases with age.1,2 This article outlines the clinical presentation and evaluation of a patient with thyrotoxicosis. Management of Graves disease, Causes of thyrotoxicosis the most frequent cause of thyrotoxicosis, is discussed in Table 1 outlines the various causes of thyrotoxicosis. The most further detail. common cause is Graves disease followed by toxic multinodular Discussion goitre, the latter increasing in prevalence with age and iodine The classic clinical manifestations of thyrotoxicosis are deficiency.3,4 Other important causes include toxic adenoma and often easily recognised by general practitioners. However, thyroiditis. Exposure to excessive amounts of iodine (eg. iodinated the presenting symptoms of thyrotoxicosis are varied, with computed tomography [CT] contrast media, amiodarone) in the atypical presentations common in the elderly. Following presence of underlying thyroid disease, especially multinodular biochemical confirmation of thyrotoxicosis, a radionuclide goitre, can cause iodine induced thyrotoxicosis. Thyroiditis thyroid scan is the most useful investigation in diagnosing is a condition that may be suitable for management in the the underlying cause. The selection of treatment differs general practice setting. Patients should be monitored for the according to the cause of thyrotoxicosis and the wishes of the hypothyroid phase, which may occur with this condition. -
Laboratory Reagents Product List 2021
PRODUCT LIST Examples of our laboratory reagents Product list – selected products Artificial Urine Brooks and Keevil AMPQ44861.1000 Auramine-Rhodamine AMPQ55029.0500 Below is a selection of products. If you cannot find what you are look- Auric Chloride 0.1% AMPQ12450.0500 ing for, please contact us about your specific requests for laboratory reagents, volume and packaging, etc. Auric Chloride 1% AMPQ12452.0100 We mainly use chemicals by p.a. quality. If you want growth control on growing media, please contact us for an offer. B Balanced Salt Solution for Storage AMPQ46214.0100 Product name Cat. No. Balanced Salt Solution with Tris AMPQ40040.1000 Barium Chloride 0.5 M = 1.0 N AMPQ42099.1000 2,4-Dinitroflouro Benzen 1.3% v/v AMPQ44913.0100 Barium Chloride 1 M AMPQ43551.0500 2-Amino-2-Methyl-1,3-propanediol 2.1 % w/v AMPQ42009.0250 Barium Chloride 10% w/v AMPQ10513.1000 2-Propanol 35% AMPQ12900.5000 Barium Diphenylamine Sulfonate AMPQ40838.0500 Basophil Counting Solution AMPQ90492.0200 A Basophilic Colouring Solution AMPQ42037.0100 Acetate Buffer 0.1 M, pH 4.0 AMPQ10021.1000 Benzamidine 0.5 M in MilliQ H2O AMPQ10750.0100 Acetate Buffer 0.1 M, pH 4.8 AMPQ40728.1000 Benzoe I Colouring Solution AMPQ10779.0100 Acetate Buffer 0.1 M, pH 5.9 AMPQ43009.1000 Benzoe II Colouring Solution AMPQ10781.0100 Acetate Buffer 35%, pH 5.6 AMPQ10015.1000 Biebrich Scarlet Solution AMPQ46088.1000 Acetate Buffer Walpole pH 4.1 AMPQ55005.0500 Biebrich's Scarlet Acid Fuchsin AMPQ29082.0500 Acetic Acid 0.1 M Titrated AMPQ11590.5000 Bies Colouring Solution AMPQ10780.0050 Acetic Acid 1% AMPQ11515.1000 BiGGY Agar AMPQ02048.0015 Acetic Acid 10% P.A. -

A Comparative Study of Aminosidine Sulfate
Iranian Journal of Pharmaceutical Research (2007), 6 (3): 209-215 Copyright © 2007 by School of Pharmacy Received: November 2005 Shaheed Beheshti University of Medical Sciences and Health Services Accepted: March 2006 Original Article A Comparative Study of Aminosidine Sulfate, Meglomine Antimoniate, Combination of both and Glucantime in Murine Leishmaniasis Treatment of Cutaneous Leishmaniasis, Caused by Leishmania tropica, with Topical Application of Paromomycin 20% in BALB-c Mice Mohammad Shahidi Dadras a*, Afshin Mirzaei b, Bahram Kazemi c, Leyla Nabai a and Ali Sharifian a aSkin Research Center, Shohada Hospital, Shaheed Beheshti University of Medical Sciences, Tehran, Iran. bFaculty of Medicine, Rafsanjan University of Medical Science, Rafsanjan, Iran. cCellular and Molecular Biology Research Center, Faculty of Medicine, Shaheed Beheshti University of Medical Sciences, Tehran, Iran. Abstract The current treatment of choice for cutaneous leishmaniasis is either parenteral or intralesional antimonial compounds. Each of these treatments has its own downfalls which include toxic side effects with the parentral injection and pain at the site of injection with the intralesional injection. In recent years, there has been more focus on Paromomycin as an alternative drug; however, current data arose many controversies. In this study, the efficacy of different therapeutic regimens including topical paromomycin 20%, topical gentamycin 0.5%, intralesional glucantime injections, topical paromomycin 20% combine with gentamycin 0.5%, and placebo were compared. The results showed that the topical application of paromomycin had better response, less recurrence. In conclusion, topical paromomycin 20% can be an appropriate substitute for intralesional injection of glucantime, but more studies are needed to support its efficacy in human cutaneous leishmaniasis. -

A Clinical Update on Vitamin D Deficiency and Secondary
References 1. Mehrotra R, Kermah D, Budoff M, et al. Hypovitaminosis D in chronic 17. Ennis JL, Worcester EM, Coe FL, Sprague SM. Current recommended 32. Thimachai P, Supasyndh O, Chaiprasert A, Satirapoj B. Efficacy of High 38. Kramer H, Berns JS, Choi MJ, et al. 25-Hydroxyvitamin D testing and kidney disease. Clin J Am Soc Nephrol. 2008;3:1144-1151. 25-hydroxyvitamin D targets for chronic kidney disease management vs. Conventional Ergocalciferol Dose for Increasing 25-Hydroxyvitamin supplementation in CKD: an NKF-KDOQI controversies report. Am J may be too low. J Nephrol. 2016;29:63-70. D and Suppressing Parathyroid Hormone Levels in Stage III-IV CKD Kidney Dis. 2014;64:499-509. 2. Hollick MF. Vitamin D: importance in the prevention of cancers, type 1 with Vitamin D Deficiency/Insufficiency: A Randomized Controlled Trial. diabetes, heart disease, and osteoporosis. Am J Clin Nutr 18. OPKO. OPKO diagnostics point-of-care system. Available at: http:// J Med Assoc Thai. 2015;98:643-648. 39. Jetter A, Egli A, Dawson-Hughes B, et al. Pharmacokinetics of oral 2004;79:362-371. www.opko.com/products/point-of-care-diagnostics/. Accessed vitamin D(3) and calcifediol. Bone. 2014;59:14-19. September 2 2015. 33. Kovesdy CP, Lu JL, Malakauskas SM, et al. Paricalcitol versus 3. Giovannucci E, Liu Y, Rimm EB, et al. Prospective study of predictors ergocalciferol for secondary hyperparathyroidism in CKD stages 3 and 40. Petkovich M, Melnick J, White J, et al. Modified-release oral calcifediol of vitamin D status and cancer incidence and mortality in men.