University of Nevada, Reno the Design and Synthesis of Monomers
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

The Use of Arynes in Organic Synthesis
Dedicated to all those too lazy to visit a library Tetrahedron 59 (2003) 701–730 Tetrahedron report number 629 The use of arynes in organic synthesis He´le`ne Pellissier* and Maurice Santelli Laboratoire de Synthe`se Organique UMR no. 6009, Faculte´ des Sciences de Saint-Je´roˆme, Avenue Esc. Normandie-Niemen, 13397 Marseille, Cedex 20, France Received 27 November 2002 Contents 1. Introduction 701 2. Structure and reactivity 701 3. Generation of arynes 702 4. Pericyclic reactions of arynes 704 4.1. Diels–Alder cycloadditions 704 4.1.1. With cyclic heterodienes 704 4.1.2. With acyclic heterodienes 707 4.1.2.1. Intermolecular cycloadditions 707 4.1.2.2. Intramolecular cycloadditions 709 4.1.3. With other dienes 709 4.2. [2þ2] Cycloadditions 711 4.3. 1,3-Dipolar cycloadditions 712 4.4. 1,4-Dipolar cycloadditions 715 4.5. Ene reactions 715 5. Nucleophilic additions to arynes 717 5.1. Addition of nitrogen nucleophiles 717 5.2. Addition of carbon nucleophiles 718 5.2.1. Lithioacetonitrile derivatives as carbon nucleophiles 718 5.2.2. Lithioenolates as carbon nucleophiles 722 6. Transition metal-catalysed reactions of arynes 723 7. Conclusions 727 1. Introduction arynes. The importance of arynes for the synthesis of natural products particularly alkaloids is well illustrated. The This review is an update of aryne chemistry and covers the term ‘arynes’ will be used to refer both to derivatives literature from 1990–2002 since early work has already of 1,2-dehydrobenzene (benzyne) and their heterocyclic been reviewed extensively.1 The preceding review covered analogues (heteroarynes). -

Fullerene-Acene Chemistry
University of New Hampshire University of New Hampshire Scholars' Repository Doctoral Dissertations Student Scholarship Spring 2007 Fullerene-acene chemistry: Part I Studies on the regioselective reduction of acenes and acene quinones; Part II Progress toward the synthesis of large acenes and their Diels-Alder chemistry with [60]fullerene Andreas John Athans University of New Hampshire, Durham Follow this and additional works at: https://scholars.unh.edu/dissertation Recommended Citation Athans, Andreas John, "Fullerene-acene chemistry: Part I Studies on the regioselective reduction of acenes and acene quinones; Part II Progress toward the synthesis of large acenes and their Diels-Alder chemistry with [60]fullerene" (2007). Doctoral Dissertations. 363. https://scholars.unh.edu/dissertation/363 This Dissertation is brought to you for free and open access by the Student Scholarship at University of New Hampshire Scholars' Repository. It has been accepted for inclusion in Doctoral Dissertations by an authorized administrator of University of New Hampshire Scholars' Repository. For more information, please contact [email protected]. FULLERENE-ACENE CHEMISTRY: PART I: STUDIES ON THE REGIOSELECTIVE REDUCTION OF ACENES AND ACENE QUINONES; PART II: PROGRESS TOWARD THE SYNTHESIS OF LARGE ACENES AND THEIR DIELS- ALDER CHEMISTRY WITH [60]FULLERENE VOLUME 1 CHAPTERS 1-5 BY ANDREAS JOHN ATHANS B.S. University of New Hampshire, 2001 DISSERTATION Submitted to the University of New Hampshire in Partial Fulfillment of the Requirements for the Degree of Doctor of Philosophy m Chemistry May, 2007 Reproduced with permission of the copyright owner. Further reproduction prohibited without permission. UMI Number: 3 2 6 0 5 8 6 INFORMATION TO USERS The quality of this reproduction is dependent upon the quality of the copy submitted. -

Synthesis of an Unsymmetrically Pentafunctionalized Corannulene Derivative (Part I) Synthesis of Platinum and Ethynyl-Platinum Corannulenes (Part II)
Zurich Open Repository and Archive University of Zurich Main Library Strickhofstrasse 39 CH-8057 Zurich www.zora.uzh.ch Year: 2012 Synthesis of an Unsymmetrically Pentafunctionalized Corannulene Derivative (Part I) Synthesis of Platinum and Ethynyl-Platinum Corannulenes (Part II) Maag, Roman M Posted at the Zurich Open Repository and Archive, University of Zurich ZORA URL: https://doi.org/10.5167/uzh-164179 Dissertation Published Version Originally published at: Maag, Roman M. Synthesis of an Unsymmetrically Pentafunctionalized Corannulene Derivative (Part I) Synthesis of Platinum and Ethynyl-Platinum Corannulenes (Part II). 2012, University of Zurich, Faculty of Science. Part I: Synthesis of an Unsymmetrically Pentafunctionalized Corannulene Derivative and Part II: Synthesis of Platinum and Ethynyl-Platinum Corannulenes Dissertation zur Erlangung der naturwissenschaftlichen Doktorwurde¨ Dr. sc. nat. vorgelegt der Mathematisch-naturwissenschaftlichen Fakult¨at der Universit¨at Zurich¨ von Roman M. Maag von Winkel ZH Promotionskommitee: Prof. Dr. Jay S. Siegel (Vorsitz) Prof. Dr. Kim K. Baldridge Prof. Dr. Cristina Nevado Prof. Dr. Roger Alberto Zurich,¨ 2012 Abstract of the Dissertation Part I: Synthesis of an Unsymmetrically Pentafunctionalized Corannulene Derivative and Part II: Synthesis of Platinum and Ethynyl-Platinum Corannulenes by Roman M. Maag University of Zurich, 2012 Prof. Dr. Jay S. Siegel, Chair Corannulene (C20H10) is a polyaromatic hydrocarbon that can be considered as the smallest fragment of Buckminsterfullerene exhibiting a curved surface. Among the in- teresting properties of corannulene are rapid bowl inversion and esthetically appealing fivefold symmetry (C5v), which is rare in chemistry. Whereas the first synthesis in 1968 only afforded milligram quantities, several improvements in the synthetic strategy finally culminated in the development of an efficient process which today furnishes corannulene in kilogram quantities. -

Mechanistic Studies Into the Reactions of Diazonium And
MECHANISTIC STUDIES INTO THE REACTIONS OF DIAZONIUM AND RELATED COMPOUNDS by CHARLES DOUGLAS MURRAY, B.Sc. Thesis presented for the degree of DOCTOR of PHILOSOPHY University of Edinburgh September 1974 GLBY TO MY PARENTS Douglas William and Isabella Seaton McKenzie Murray • • AND MY BROTHER • • William Graeme Murray I declare that this thesis is my own composition, that the work of which it is a record has been carried.out by myself, and that it has not been submitted in any previcis application for a Higher Degree. The thesis describes results of research carried out • in the Department of Chemistry, University of Edinburgh under the supervision of Professor J. I. G. Cadogan since the 1st October, • 1971, the date of my admission as a research student. The following is a statement of postgraduate courses attended during the last three years: Summer School in Mass Spectrometry, University of Sheffield, 26-30 March 1972; - Recent Developments in the Theory of Concerted Processes, Dr. A. J. Bellamy (five lectures); Organometallic Processes in Organic Chemistry, Professor P. L. Pauson (five lectures); Industrial Research and Development, Dr. B. Gravenor (five lectures); E. U. Chemistry Department Seminars (twenty-five periods). • ACKNOWLEDGEMENTS I should like to express my gratitude to Professor J. I. G. Cadogan for suggesting the topic of research and to Professor Cadogan and Dr. J. T. Sharp for their guidance and encouragement in all aspects of this work. I would also like to record my thanks and appreciation to various other members of Edinburgh University Chemistry Department: to Dr. R. M.Paton for advice and assistance related to e. -

Sulfolane: Magic Extractor Or Bad Actor? Pilot-Scale Study on Solvent Corrosion Potential
sustainability Review Sulfolane: Magic Extractor or Bad Actor? Pilot-Scale Study on Solvent Corrosion Potential Andrzej Bak 1,* , Violetta Kozik 1, Paulina Dybal 1, Slawomir Kus 2, Aleksandra Swietlicka 1 and Josef Jampilek 3,* 1 Department of Synthesis Chemistry, Faculty of Mathematics, Physics and Chemistry, University of Silesia, Szkolna 9, 40007 Katowice, Poland; [email protected] (V.K.); [email protected] (P.D.); [email protected] (A.S.) 2 Honeywell Process Solutions, 11201 Greens Crossing Blvd, Suite 700 Houston, TX 77067, USA; [email protected] 3 Department of Pharmaceutical Chemistry, Faculty of Pharmacy, Comenius University, Odbojarov 10, 83232 Bratislava, Slovakia * Correspondence: [email protected] (A.B.); [email protected] (J.J.); Tel.: +48-32-359-1197 (A.B.) Received: 17 September 2018; Accepted: 11 October 2018; Published: 14 October 2018 Abstract: The sulfur-containing derivatives and their metabolites, regarded as ‘old devils of green’ chemistry, constitute a relevant class of air/water/soil contaminants in over-polluted world. In fact, some industrially-engineered solvents have become environmentally unfavorable. An attractive alternative to commonly used industrial liquids is sulfolane (C4H8SO2), an anthropogenic medium. The main objective of this paper is the comprehensive review focusing mainly on the state-of-the-art aspects of the sulfolane synthesis, application of sulfolane as an extractive solvent due to its ‘unique’ physicochemical properties as well as the potential of sulfolane to cause equipment corrosion and subsequent spills. The potential risk for groundwater contamination, danger for human health and ways of sulfolane biodegradation were briefly reviewed as well. Interestingly, the analysis performed on data stored in the Reaxys database revealed an alternating tendency of waxing and waning interest in sulfolane during the space of the last fifty years. -

Molecular Assembly of Polycyanoarenes with Silver Salts
MOLECULAR ASSEMBLY OF POLYCYANOARENES WITH SILVER SALTS AND SYNTHESIS OF POLYCYCLIC AROMATIC HYDROCARBONS A DISSERTATION IN Chemistry and Pharmaceutical Sciences Presented to the Faculty of the University of Missouri-Kansas City in partial fulfillment of the requirements for the degree DOCTOR OF PHILOSOPHY by GERARDO B. MÁRQUEZ B.Sc. Central University of Venezuela, 1993 Kansas City, Missouri 2012 © 2012 GERARDO B. MÁRQUEZ ALL RIGHTS RESERVEDED MOLECULAR ASSEMBLY OF POLYCYANORENES WITH SILVER SALTS AND SYNTHESIS OF POLYCYCLIC AROMATIC HYDROCARBONS Gerardo Batalla Márquez, Candidate for the Doctor of Philosophy Degree University of Missouri-Kansas City, 2012 ABSTRACT This dissertation encompasses the investigation of two distinct subjects. In the first part, which is in the area of molecular self-assembly, the complexation of organonitrile aryl compounds with three different types of silver (I) salts is examined in the solid state. The assembly of 1-(2,2-dicyanovinyl)naphthalene with silver hexafluoroantimonate resulted in a cationic 3D network. Complexation of 4-(2,2-dicyanovinyl)biphenyl with silver tetrafluoroborate and hexafluoroantimonate from benzene generated two similar structures. While the former displays a cationic 3D network, the latter is defined by cationic 2D sheets. Complexation of 9-(2,2-dicyanovinyl)anthracene with silver hexafluoroantimonate from toluene afforded a cationic 2D ribbon, and from benzene, it yielded cationic 2D sheets. These complexes contained solvent bonded to their structures. However, the hexafluoroantimonate ion is nonbonding. The crystal association of 1,4-bis(cyanovinyl)benzene with silver triflate from benzene yielded neutral 2D sheets whose imperfect-rectangular macrocyclic arrangements are interconnected on both sides by bridges of benzene. On the other hand, the assembly of 1,3- bis(cyanovinyl)benzene with silver triflate from benzene afforded a neutral 3D network formed by two interconnected rings. -

Anti‐Aromatic Versus Induced Paratropicity ... -.:. Michael Pittelkow
Angewandte Forschungsartikel Chemie Deutsche Ausgabe:DOI:10.1002/ange.201913552 Circulenes Internationale Ausgabe:DOI:10.1002/anie.201913552 Anti-Aromatic versus Induced Paratropicity:Synthesis and InterrogationofaDihydro-diazatrioxa[9]circulene with aProton Placed Directly above the Central Ring Stephan K. Pedersen, Kristina Eriksen, Nataliya N. Karaush-Karmazin, Boris Minaev, Hans gren, Gleb V. Baryshnikov* und Michael Pittelkow* Abstract: We present ahigh-yielding intramolecular oxidative coupling within adiazadioxa[10]helicene to give adihydro- diazatrioxa[9]circulene.This is the first [n]circulene contain- ing more than eight ortho-annulated rings (n > 8). The single- crystal X-raystructure reveals atight columnar packing, with aproton from apendant naphthalene moiety centred directly abovethe central nine-membered ring. This distinct environ- ment induces asignificant magnetic deshielding effect on that particular proton as determined by 1HNMR spectroscopy. The origin of the deshielding effect was investigated computation- ally in terms of the NICS values.Itisestablished that the deshielding effect originates from an induced paratropic ring current from the seven aromatic rings of the [9]circulene structure,and is not due to the nine-membered ring being antiaromatic.UV/Vis spectroscopyreveals more efficient Figure 1. Simplified illustration of the influence of diatropic and para- conjugation in the prepared diazatrioxa[9]circulene compared tropic ring currents on the 1Hchemical shift inside and outside of to the parent helical azaoxa[10]helicenes,and -

Synthesis of Functionalized Benzenoid Macrocycles: an Approach to Accessing Functionalized Cycloparaphenylenes
Synthesis of Functionalized Benzenoid Macrocycles: An Approach to Accessing Functionalized Cycloparaphenylenes by Rolande Meudom A thesis submitted to the Graduate Faculty of Auburn University in partial fulfillment of the requirements for the Degree of Master of Science Auburn, Alabama May 8, 2016 Keywords: Carbon nanotubes, Cycloparaphenylenes, p-terphenylophanes, Bent p-phenylene Macrocycles, Copyright 2016 by Rolande Meudom Approved by Bradley L. Merner, Chair, Assistant Professor of Chemistry and Biochemistry Peter Livant, Associate Professor of Chemistry and Biochemistry Anne E. V. Gorden, Associate Professor of Chemistry and Biochemistry Steven Mansoorabadi, Assistant Professor of Chemistry and Biochemistry Abstract Carbon nanotubes (CNTs) are cylindrically shaped allotropes of carbon with exceptionally good electronic and optical properties. The current state of art for making these CNTs involves heating graphite in a high temperature furnace (above 600 °C). The use of harsh temperatures makes the process unselective and produces CNTs as an inseparable mixture of products. Currently, there is no efficient purification technique for isolating pure CNTs. In order to fully exploit the electronic properties of CNTs, they need to be made as homogeneous and monodispersed structures. Thus, synthetic chemists envisioned using chemical synthesis from the ground-up via a template strategy as a more viable approach to achieve the selective synthesis of uniform CNTs structures with a defined chirality and diameter. This is because the selective synthesis of curved PAHs like [n]ciruclenes and CNT substructures could only be accomplished through chemical synthesis. The work described herein focuses on a new approach to accessing highly distorted p-phenylenes, which does not rely on cross coupling reactions and the use of the Burgess reagent as a mild dehydrative aromatization agent, giving access to highly strained benzenoid macrocycles. -
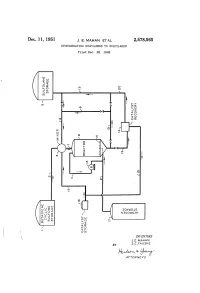
Rudo-At-Thur a 77OAPAVA 1S Patented Dec
Dec. 11, 1951 J. E. MAHAN ET AL 2,578,565 HYDROGENATING SULFOLENES TO SULFOLANES Filed Dec. 28, 1948 o g X g t 92.9 DVOS a dig NGOOOH Ofse INVENTORS J.E. MAHAN BY S.C. FAUSKE Rudo-at-thur A 77OAPAVA 1S Patented Dec. 11, 1951 2,578,565 UNITED STATES PATENT OFFICE 2,578,565 HYDROGENATING SULFOLENESTO SULFOLANES John E. Mahan and Sig C. Fauske, Bartlesville, Okla., assignors to Phillips Petroleum Company, a corporation of Delaware Application December 28, 1948, Serial No. 67,745 15 Claims. (CI. 260-332.1) 1. 2 This invention relates to the production of the generic term “a sulfolane' or "a Sulfolane Sulfolanes by the hydrogenation of the corre compound' covering not only the above Com sponding unsaturated Sulfolenes. In One par pound but also the substituted derivatives there ticular embodiment it relates to an improved of, particularly those in which various radicals process for the production of 2,3,4,5-tetrahy mentioned in the preceding paragraph are Sub drothiophene-1,1-dioxide, commercially known stituted for one or more of the hydrogen atoms as sulfolane, by the catalytic hydrogenation of of the above structure. Where such a radical the corresponding unsaturated cyclic butadiene is hydrogenatable under the conditions of our monosulfone, i. e. 2,5-dihydrothiophene-1,1- process, it will be understood that the sulfolane dioxide, commercially known as Sulfolene, in the 0. containing the hydrogenated radical is included presence of a novel solvent. when reference is made to a sulfolane compound The term “a sulfolene compound' as employed which “corresponds' to a given sulfolene com herein and in the appended claims, defines ge pound. -
![[7]Helicene to a Planar Hetero[8]Circulene Bodil Lousen,[A] Stephan K](https://docslib.b-cdn.net/cover/9648/7-helicene-to-a-planar-hetero-8-circulene-bodil-lousen-a-stephan-k-1969648.webp)
[7]Helicene to a Planar Hetero[8]Circulene Bodil Lousen,[A] Stephan K
Communication Chemistry—A European Journal doi.org/10.1002/chem.201905339 & Non-Planar Aromatics Compressing aNon-Planar Aromatic Heterocyclic [7]Helicene to a Planar Hetero[8]Circulene Bodil Lousen,[a] Stephan K. Pedersen,[a] Pernille Bols,[a] Kasper H. Hansen,[a] MichelleR.Pedersen,[a] Ole Hammerich,[a] Sergey Bondarchuk,[b] Boris Minaev,[b] Glib V. Baryshnikov,[c] Hans gren,[c, d] and MichaelPittelkow*[a] cally chiral moleculeshave found vast applications in such di- Abstract: This work describes asyntheticapproachwhere verse fields as molecular recognition,catalysis anddetection or anon-planar aromatic heterocyclic [7]helicene is com- emissionofcircularlypolarized light.[2–5] Structurally related pressedtoyield ahetero[8]circulene containing an inner motifstothe [n]helicenes are the [n]circulenes. These struc- antiaromatic cyclooctatetraene (COT) core. This [8]circu- tures also consist of ortho-annulatedaromatic rings, but in- lene consists of four benzene rings and four heterocyclic stead forms acyclic structure, having acentral ring with n rings, and it is the first heterocyclic[8]circulenecontaining sides.Itisillustrative to view the all-benzene series of [n]circu- three differentheteroatoms. The synthetic pathway pro- lenes,which consistsofthe bowl-shaped carba[5]circulene ceeds via athe flattened dehydro-hetero[7]helicene, (corannulene), the planar carba[6]circulene(coronene) andthe which is partially ahelicene and partially acirculene:itis saddle-shaped[7]- and [8]circulenes. Hetero[8]circulenes incor- non-planar and helically chiral as helicenes, and contains a poratingfour five-membered heterocycles(furans,pyrroles, COT motif like [8]circulenes. The antiaromaticity of the thiophenes)are planar,[6,7] and these structures can be viewed COT core is confirmed by nucleusindependent chemical as containingantiaromaticcyclooctatetraene(COT) cores. -

Arynes in Synthesis; New Reaction and Precursor Development
ARYNES IN SYNTHESIS; NEW REACTION AND PRECURSOR DEVELOPMENT A thesis submitted to the University of Manchester for the degree of Doctor of Philosophy in the Faculty of Engineering and Physical Sciences 2014 By Donald McAusland School of Chemistry 2 Contents Abstract 11 Declaration 12 Copyright 13 Acknowledgements 14 1 Introduction 15 1.1 Generationofbenzyne ............................ 17 1.2 Reactionsofbenzyne............................. 19 1.2.1 Pericyclicreactions. 19 1.2.2 Nucleophilicadditiontoarynes . 21 1.2.3 Transition-metal-catalysed reactions . 26 1.3 Selectivitywithsubstitutedarynes . ..... 28 1.4 (Trimethylsilyl)phenyl triflates and related aryne precursors . 32 1.4.1 ortho-Lithiation ........................... 33 1.4.2 [4+2]Cycloaddition . 34 1.4.3 Oxidative para-triflation of acetanilides . 34 1.4.4 Commerciallyavailableprecursors . 35 1.4.5 Precursors for the synthesis of polyaromatic structures...... 35 1.4.6 Other functionalised Kobayashi precursors . ..... 37 1.4.7 Relatedaryneprecursors. 39 1.5 Arynesinsynthesis.............................. 41 1.5.1 Indolesyntheses ........................... 42 2 The Benzyne Fischer Indole Reaction 46 2.1 Introduction.................................. 46 2.1.1 Buchwaldmodification. 47 Contents 3 2.1.2 The arylation of hydrazones with benzyne . 52 2.2 Application of aryne electrophiles to the Fischer indole synthesis . 52 2.2.1 Aryne reactions of other hydrazones . 55 2.2.2 BenzyneFischerindolescope . 58 2.2.3 Substitutedarynes . 60 2.2.4 Miscellaneoustosylhydrazones. 66 2.3 Conclusions .................................. 67 3 Synthesis and Applications of New Aryne Precursors 68 3.1 Introduction.................................. 68 3.1.1 Palladium cross-coupling chemistry . 68 3.1.2 TheSuzukireaction ......................... 69 3.1.3 Organoboronreagents . 71 3.1.4 Chemoselectivity in Suzuki couplings . 74 3.2 Aims..................................... -
Photochemical Reactions As Key Steps in Natural Product Synthesis Thorsten Bach* and J�Rg P
Reviews T. Bach and J. P. Hehn DOI: 10.1002/anie.201002845 Synthetic Methods Photochemical Reactions as Key Steps in Natural Product Synthesis Thorsten Bach* and Jrg P. Hehn Keywords: Dedicated to Professor David A. Evans cyclization · cycloaddition · on the occasion of his 70th birthday natural products · photochemistry · total synthesis Angewandte Chemie 1000 www.angewandte.org 2011 Wiley-VCH Verlag GmbH & Co. KGaA, Weinheim Angew. Chem. Int. Ed. 2011, 50, 1000 – 1045 Photochemical Reactions Photochemical reactions contribute in a significant way to the existing From the Contents repertoire of carbon–carbon bond-forming reactions by allowing access to exceptional molecular structures that cannot be obtained by 1. Introduction 1001 conventional means. In this Review, the most important photochemical 2. Photocyclizations 1001 transformations that have been employed in natural product synthesis are presented. Selected total syntheses are discussed as examples, with 3. Norrish–Yang Cyclizations 1008 particular attention given to the photochemical key step and its ste- reoselectivity. The structural relationship between the photochemically 4. Norrish Type I Cleavage Reactions 1009 generated molecule and the natural product is shown, and, where necessary, the consecutive reactions in the synthesis are illustrated and 5. Photochemical classified. Rearrangements 1011 6. Reactions via Dienol Intermediates 1015 1. Introduction 7. Patern–Bchi Reaction 1017 Is there anything that hasnt already been said or written about natural product synthesis?[1] Great art has been seen in 8. [2+2] Photocycloadditions of it,[2] and attempts have been made to establish it as a Olefins 1018 handcraft. Economical rules have been assigned to it,[3] and it has been fitted into logical schemes.[4] Some people view 9.