Thirty Years of Disdain
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Beyond Myalgic Encephalomyelitis/Chronic Fatigue Syndrome: Redefining an Illness
This PDF is available from The National Academies Press at http://www.nap.edu/catalog.php?record_id=19012 Beyond Myalgic Encephalomyelitis/Chronic Fatigue Syndrome: Redefining an Illness ISBN Committee on the Diagnostic Criteria for Myalgic 978-0-309-31689-7 Encephalomyelitis/Chronic Fatigue Syndrome; Board on the Health of Select Populations; Institute of Medicine 330 pages 6 x 9 PAPERBACK (2015) Visit the National Academies Press online and register for... Instant access to free PDF downloads of titles from the NATIONAL ACADEMY OF SCIENCES NATIONAL ACADEMY OF ENGINEERING INSTITUTE OF MEDICINE NATIONAL RESEARCH COUNCIL 10% off print titles Custom notification of new releases in your field of interest Special offers and discounts Distribution, posting, or copying of this PDF is strictly prohibited without written permission of the National Academies Press. Unless otherwise indicated, all materials in this PDF are copyrighted by the National Academy of Sciences. Request reprint permission for this book Copyright © National Academy of Sciences. All rights reserved. Beyond Myalgic Encephalomyelitis/Chronic Fatigue Syndrome: Redefining an Illness Beyond Myalgic Encephalomyelitis/ Chronic Fatigue Syndrome Redefining an Illness Committee on the Diagnostic Criteria for Myalgic Encephalomyelitis/Chronic Fatigue Syndrome Board on the Health of Select Populations PREPUBLICATION COPY—Uncorrected Proofs Copyright © National Academy of Sciences. All rights reserved. Beyond Myalgic Encephalomyelitis/Chronic Fatigue Syndrome: Redefining an Illness THE NATIONAL ACADEMIES PRESS 500 Fifth Street, NW Washington, DC 20001 NOTICE: The project that is the subject of this report was approved by the Govern- ing Board of the National Research Council, whose members are drawn from the councils of the National Academy of Sciences, the National Academy of Engineer- ing, and the Institute of Medicine. -

Sensationalism Versus Science?
Sensationalism versus Science? Malcolm Hooper and Margaret Williams 5th May 2013 The long-promised article by Michael Hanlon on the “war” surrounding the neuroimmune disorder myalgic encephalomyelitis (ME) was published today in the Sunday Times Magazine together with a photograph of Professor Sir Simon Wessely (“This man faced death threats and abuse. His crime? He suggested that ME was a mental illness”). Whilst the article focuses on the unacceptable actions of very few alleged sufferers and not on legitimate challenges mounted by scientists, it may be of interest to reproduce the questions which Hanlon sent to Professor Malcolm Hooper on 31st January and also Professor Hooper’s reply, since that reply provides the backdrop to the on-going “war”. First, though, it must be said that much of Hanlon’s article is factually wrong. For example, Hanlon begins his article with the assertion that “Professor Sir Simon Wessely lives on the front line of science”,which immediately reveals a lack of understanding in that Sir Simon does not live on the front line of biomedical science but on the front line of psychiatry, a discipline with no biomarkers of disease. Indeed Sir Simon ignores, actively denies or dismisses the evidence of biomedical scientists that demonstrates whole-body inflammation in ME and does not accept that it has now been shown to be an autoimmune disease. Hanlon goes on to state that Sir Simon “has done a lot of work for the military, helping to treat traumatised war veterans”, when there is irrefutable published evidence that Sir Simon has for decades denied the very existence of Gulf War Syndrome. -

New ME Briefing 21 J
1 Briefing for ‘Myalgic Encephalomyelitis: Treatment st and Research’ Westminster Hall, 21 June 2018 Contents About ME 3 The Debate 3 Education of health professionals 4 Review of the NICE guideline on ME/CFS 5 NHS services 6 Children and young people with ME 7 Severe ME 7 Biomedical research 8 PACE trial 10 ME and welfare benefits 10 Social Care 11 References 13 Contact details 15 Appendix: The PACE Trial Controversy 16 This briefing has been produced jointly by #MEAction, Action for M.E., ME Association and the ME Trust (for contact details, see page 15) Supported by: Blue Ribbon for Awareness of ME, Centre for Welfare Reform, Forward-ME, ME Research UK, WAMES 2 A bout ME Myalgic Encephalomyelitis (ME) is a chronic, fluctuating, neurological condition that causes symptoms affecting many body systems, more commonly the nervous and immune systems. ME affects an estimated 250,000 adults and children in the UK, and around 17 million people worldwide. People with ME experience severe, persistent fatigue associated with post-exertional malaise (PEM), their systems’ inability to recover after expending even small amounts of energy, leading to a flare-up in symptoms. PEM means that simple mental or physical activities can leave people with ME debilitated, experiencing a range of symptoms that are not significantly relieved by resting. PEM is the hallmark symptom of ME, other symptoms can include muscle and joint pain, cognitive difficulties, noise and light sensitivities and digestive problems. People with ME can vary enormously in their experience of the illness, and how their symptoms fluctuate. An estimated one in four children and adults with ME experience these symptoms severely (see ‘Severe ME’ on page 7). -

Chronic Fatigue Syndrome/Myalgic
# 13 – October 2015 1. Colofon / Personalia Scientific reviews: Richard Podell Advisory board: Leonard A. Jason Cartoons: Djanko Editor/Editorial team: David Egan, Eddy Keuninckx, Rob Wijbenga Textual contributions for the December-issue: Prof. Alan Light John Allsop Annet Lindhout Prof. Leonard Jason Bente Stenfalk Lieke Kops Brenda Vreeswijk Lisa Forstenius Dr. Byron Hyde Maartje Slot Carin Widholm Merry S. CJ Arlotta Michael Evison David Egan Dr. Neil Abbott Eddy Keuninckx P.E. Griffith Erica Verrillo Rebecca Hansen Faith Wong Dr. Richard Podell Greg & Linda Crowhurst Rob Wijbenga Helle Rasmussen Rosemary Underhill Jeannette Burmeister Ryan Prior Jerrold Spinhirne Sally K. Burch Jeryldine Saville Scott Jordan Harris Jo Best Staffan Christensen Joan McParland Distribution: Eddy Keuninckx Layout: Eddy Keuninckx Archive: http://let-me.be (here you can download all magazines for free) Facebook: https://www.facebook.com/groups/TheMEGlobalChronicle/ The editorial team doesn't accept any responsibility for any possible incorrect information that it has been supplied with and which has been published in this monthly issue. Anyone can subscribe to this free magazine by sending their email address to: [email protected] You can un-subscribe by sending a mail to: [email protected] Textual contributions for the October issue need to be supplied in Word by 10st December and sent to: [email protected] The next issue will come out on December 20nd,2015. Subscribe to this newsletter We are no association or society, just a bunch of idealists who want to give our best efforts towards recognition of this terrible disease. By trying to help connecting to each other all patients all over the world. -

Straight-Jacketed by Empty Air
Straight-jacketed by empty air Psychiatry’s long and shameful involvement in Myalgic Encephalomyelitis St Straight-jacketed by empty air Greg Crowhurst Please note that the publisher and the author cannot be held accountable for any damages or actions arising from reading this book, which is presented for informational purposes only. It is not intended as a substitute for professional medical advice. The views of contributors are not necessarily those of the publisher or author, under no circumstances can they be held accountable for any loss or claim arising out of the opinions expressed or suggestions made. “There is no doubt that ME is an organic disorder. The sufferers are denied proper recognition, misdiagnosed, vilified, ridiculed and driven to great depths of despair.” Hansard (House of Commons) 23rd February 188/columns 167-168 Books by Greg Crowhurst : Kevin Mayhew : St Pauls : Holy Way , Stonebird S Stonebird Holy Way Dedication I dedicate this, with all the love in my heart to my wife Linda, whose agony is beyond imagination or comprehension. All I have to help are these arms to reach out, hold and comfort, but that is more and more unbearable for her to cope with, so severe is her torture. Acknowledgements: Many, many thanks for all the support and enthusiasm for this book, it spurred me on! Special thanks to Rachel Higginson, Susanna Agardy, Geoffrey Keith Brown, Sally K Burch, Colleen Steckel, Nancy Blake, Corina Duyn, Clare Norton, Naomi Whittingham and Theresa Brennan. “I must emphasise that the most important message -
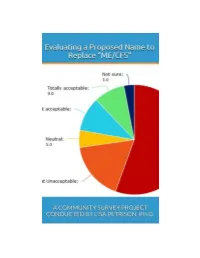
Read the Full Survey Report
Evaluating a Proposed Name to Replace ‘ME/CFS’: A Community Survey Project Conducted by Lisa Petrison, Ph.D. Published by Paradigm Change (March 2015) Copyright 2015 www.paradigmchange.me 2 Table of Contents Executive Summary ...................................................................................................................................... 5 Part 1 – Results Overview .......................................................................................................................... 13 Part 2 - Implications ................................................................................................................................... 29 Part 3 – Survey Questions .......................................................................................................................... 49 Part 4 – Main Survey Results ..................................................................................................................... 66 Part 5 – ME vs. Non-ME Results ............................................................................................................... 110 Part 6 – US ME vs. Non-ME Results ......................................................................................................... 127 Part 7 – Related Illnesses Results ............................................................................................................ 161 Part 8 – US vs. Non-US Results ................................................................................................................. 181 -

The Biopolitics of Chronic Fatigue Syndrome
The Biopolitics of Chronic Fatigue Syndrome Thesis submitted for the degree of Doctor of Philosophy at the University of Leicester by Nikos Karfakis School of Management University of Leicester March 2013 Abstract The Biopolitics of Chronic Fatigue Syndrome by Nikos Karfakis This thesis approaches Chronic Fatigue Syndrome (CFS) as a biopolitical problem, that is as a shifting scientific object which needs to be studied, classified and regulated. Assemblages of authorities, knowledges, and techniques make CFS subjects and shape their everyday conduct in an attempt to increase their supposed autonomy, wellbeing and health. CFS identities are, however, made not only through government, scientific and medical interventions but also by the patients themselves, a biosocial community that collaborates with scientists, educates itself about the intricacies of biomedicine, and contests psychiatric truth claims. CFS is a socio-medical disorder, an illness trapped between medicine, psychology and society, an illness that is open to debate, and therefore difficult to manage and standardise. CFS is, thus, more than a fixed and defined medical category; it is a performative and multiple category, it is a heterogeneous world. This thesis studies that performative complexity by assembling different pieces of empirical data that constitute its heterogeneity: medical and psychiatric journals and monographs, self-help books, CFS organisations’ magazines, newsletters and websites, illness narratives and social studies of CFS, CFS blogs, and qualitative interviews with diagnosed CFS patients and CFS activists. The thesis delineates different interventions by medicine, science, the state and the patients themselves and concludes that CFS remains elusive, only partially standardised, in an on- going battle between all the different actors that want to define it for their own situated interests. -

Journal of Iime Volume 1 Issue 1 2
JJJooouuurrrnnnaaalll ooofff IIIiiiMMMEEE IIInnnvvveeesssttt iiinnn MMMEEE IIInnnttteeerrrnnnaaatttiiiooonnnaaalll MMMEEE///CCCFFFSSS CCCooonnnfffeeerrreeennnccceee 222000000777 SSSpppeeeccciiiaaalll VOLUME 1 ISSUE 1 Invest in ME Charity Nr 1114035 www.investinme.org Journal of IiME Volume 1 Issue 1 2 Welcome from Invest in ME Welcome to the first Journal of Invest in ME – a combination of research, information, news, stories and other articles relating to myalgic encephalomyelitis (ME). Facts About ME This first version is also serving a dual purpose in that it is acting as a An estimated 250,000 component of the delegate’s conference pack for the 2nd Invest in ME International ME/CFS Conference 2007, held in Westminster, London, UK. people suffer from ME in the UK Invest in ME welcomes delegates, presenters and media from ten countries around the world to the conference – emphasising that ME recognises no international boundaries. The interest in the conference also demonstrates the need for some of IiME’s main objectives – more education and proper funding for biomedical research into ME. In this document we include articles from renowned ME experts who ME Story were not able to be present at the conference this year as well as those who are. We also include other ME experts who are presenting. IiME I fell ill with the flu the hope to publish our journal throughout the year. As with the IiME same time as Antony, conference it will allow a platform for researchers, scientists, healthcare my then staff and politicians to be able to share and provide information boyfriend/fiancé. He relevant to those supporting, campaigning for or suffering from ME. -

Parliamentary Briefing
1 BRIEFING FOR MYALGIC ENCEPHALOMYELITIS: 'TREATMENT AND RESEARCH' WESTMINSTER HALL, 21 JUNE 2018. (This briefing has been reproduced without amendment for the Back Bench Business debate on 24 Jan 2019) Contents About ME 3 Education of health professionals 4 Review of the NICE guideline on ME/CFS 5 NHS services 6 Children and young people with ME 7 Severe ME 7 Biomedical research 8 PACE trial 10 ME and welfare benefits 10 Social Care 11 References 13 Contact details 15 Appendix: The PACE Trial Controversy 16 This briefing has been produced jointly by #MEAction, Action for M.E., ME Association and the ME Trust (for contact details, see page 15) Supported by: Blue Ribbon for Awareness of ME, Centre for Welfare Reform, Forward-ME, ME Research UK, Tymes Trust, WAMES, Hope 4 ME & Fibro Northern Ireland, 25% M.E. Group 2 About ME Myalgic Encephalomyelitis (ME) is a chronic, fluctuating, neurological condition that causes symptoms affecting many body systems, more commonly the nervous and immune systems. ME affects an estimated 250,000 adults and children in the UK, and around 17 million people worldwide. People with ME experience severe, persistent fatigue associated with post-exertional malaise (PEM), their systems’ inability to recover after expending even small amounts of energy, leading to a flare-up in symptoms. PEM means that simple mental or physical activities can leave people with ME debilitated, experiencing a range of symptoms that are not significantly relieved by resting. PEM is the hallmark symptom of ME, other symptoms can include muscle and joint pain, cognitive difficulties, noise and light sensitivities and digestive problems. -
CBT and GET Database
www.hfme.org/cbtandget.htm A CBT and GET database CBT and GET database Copyright © Jodi Bassett September 2006. This version updated April 2009. Taken from www.hfme.org The CBT and GET Database is a stand-alone comprehensive guide to the use of CBT and GET on patients with Myalgic Encephalomyelitis (M.E.) and the bogus psychiatric or 'behavioural' paradigm of M.E. generally. This 170 + page resource is aimed at lawyers, politicians, media, the friends and family of sufferers - but primarily at those clinicians who choose to recommend CBT and GET to their patients. It is hoped that these doctors will read something here that will forever change their minds on this subject and so benefit their patients, and themselves, as well as society in general. TABLE OF CONTENTS: Page 3 Section 1: Introduction/Overview: Features the ‗Smoke and Mirrors‘ paper and includes a copy of ‗The effects of CBT and GET on patients with Myalgic Encephalomyelitis‘ Page 43 Section 2: Recommended background reading. Includes a copy of ‗A warning on ‗CFS,‘ ‗ICD- CFS‘ and ‗ME/CFS‘ research and advocacy‘ and ‗What is M.E.?‘ Page 69 Section 3: Research and articles which expose the lack of scientific legitimacy (and the hidden financial and political motivations) underlying the 'behavioural' paradigm of M.E. and the use of CBT and GET on M.E. patients Page 122 Section 4: A summary of the available medical research Page 132 Section 5: Patient accounts of CBT Page 153 Section 6: Patient accounts of GET Page 177 Section 7: Conclusion/Summary of key points (followed by a full reference list) www.hfme.org 2 A CBT and GET database Smoke and mirrors - An analysis of the scientific legitimacy of the claims that cognitive behavioural therapy (CBT) and graded exercise therapy (GET) are appropriate, safe and effective treatments for people with M.E. -

Briefing for ‘Myalgic Encephalomyelitis: Treatment St and Research’ Westminster Hall, 21 June 2018
1 Briefing for ‘Myalgic Encephalomyelitis: Treatment st and Research’ Westminster Hall, 21 June 2018 Contents About ME 3 The Debate 3 Education of health professionals 4 Review of the NICE guideline on ME/CFS 5 NHS services 6 Children and young people with ME 7 Severe ME 7 Biomedical research 8 PACE trial 10 ME and welfare benefits 10 Social Care 11 References 13 Contact details 15 Appendix: The PACE Trial Controversy 16 This briefing has been produced jointly by #MEAction, Action for M.E., ME Association and the ME Trust (for contact details, see page 15) Supported by: Blue Ribbon for Awareness of ME, Centre for Welfare Reform, Forward-ME, ME Research UK, WAMES, Hope 4 ME & Fibro Northern Ireland 2 A bout ME Myalgic Encephalomyelitis (ME) is a chronic, fluctuating, neurological condition that causes symptoms affecting many body systems, more commonly the nervous and immune systems. ME affects an estimated 250,000 adults and children in the UK, and around 17 million people worldwide. People with ME experience severe, persistent fatigue associated with post-exertional malaise (PEM), their systems’ inability to recover after expending even small amounts of energy, leading to a flare-up in symptoms. PEM means that simple mental or physical activities can leave people with ME debilitated, experiencing a range of symptoms that are not significantly relieved by resting. PEM is the hallmark symptom of ME, other symptoms can include muscle and joint pain, cognitive difficulties, noise and light sensitivities and digestive problems. People with ME can vary enormously in their experience of the illness, and how their symptoms fluctuate. -

NAM Chronic Fatigue Syndrome
This PDF is available from The National Academies Press at http://www.nap.edu/catalog.php?record_id=19012 Beyond Myalgic Encephalomyelitis/Chronic Fatigue Syndrome: Redefining an Illness ISBN Committee on the Diagnostic Criteria for Myalgic 978-0-309-31689-7 Encephalomyelitis/Chronic Fatigue Syndrome; Board on the Health of Select Populations; Institute of Medicine 330 pages 6 x 9 PAPERBACK (2015) Visit the National Academies Press online and register for... Instant access to free PDF downloads of titles from the NATIONAL ACADEMY OF SCIENCES NATIONAL ACADEMY OF ENGINEERING INSTITUTE OF MEDICINE NATIONAL RESEARCH COUNCIL 10% off print titles Custom notification of new releases in your field of interest Special offers and discounts Distribution, posting, or copying of this PDF is strictly prohibited without written permission of the National Academies Press. Unless otherwise indicated, all materials in this PDF are copyrighted by the National Academy of Sciences. Request reprint permission for this book Copyright © National Academy of Sciences. All rights reserved. Beyond Myalgic Encephalomyelitis/Chronic Fatigue Syndrome: Redefining an Illness Beyond Myalgic Encephalomyelitis/ Chronic Fatigue Syndrome Redefining an Illness Committee on the Diagnostic Criteria for Myalgic Encephalomyelitis/Chronic Fatigue Syndrome Board on the Health of Select Populations PREPUBLICATION COPY—Uncorrected Proofs Copyright © National Academy of Sciences. All rights reserved. Beyond Myalgic Encephalomyelitis/Chronic Fatigue Syndrome: Redefining an Illness THE NATIONAL ACADEMIES PRESS 500 Fifth Street, NW Washington, DC 20001 NOTICE: The project that is the subject of this report was approved by the Govern- ing Board of the National Research Council, whose members are drawn from the councils of the National Academy of Sciences, the National Academy of Engineer- ing, and the Institute of Medicine.