Study Protocol
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Canada Leads the Global Cannabis Paradigm Shift Initiating Aphria and Canopy at Outperform
Canada Leads the Global Cannabis Paradigm Shift Initiating Aphria and Canopy at Outperform May 2018 Tamy Chen, CFA Peter Sklar, CPA, CA Cannabis Analyst Retailing/Consumer Analyst BMO Nesbitt Burns Inc. BMO Nesbitt Burns Inc. (416) 359-5501 (416) 359-5188 [email protected] [email protected] This report is intended for Canadian & EU distribution only. Unauthorized reproduction, transmission or publication without the prior written consent of BMO Capital Markets is strictly prohibited. This report was prepared by an analyst(s) employed by BMO Nesbitt Burns Inc., and who is (are) not registered as a research analyst(s) under FINRA rules. For disclosure statements, including Analyst’s Certification, please refer to pages 50 to 53. 16:00 ET~ Table of Contents Initiating BMO Cannabis Coverage ................................................................................................................... 2 Executive Summary .......................................................................................................................................... 4 Legal Environment Favours Canadian LPs ........................................................................................................ 6 Initial Recreational Market Outlook ................................................................................................................. 7 Current Medical Market in Canada: Opaque .................................................................................................. 13 Near-Term International Medical Opportunity: -

TOP NEWS • in World First, UK Approves Pfizer-Biontech COVID
TOP NEWS • In world first, UK approves Pfizer-BioNTech COVID-19 vaccine Britain approved Pfizer's COVID-19 vaccine, jumping ahead of the United States and Europe to become the West's first country to formally endorse a jab it said should reach the most vulnerable people early next week. • RBC profit beats estimates on capital markets strength, lower provisions Royal Bank of Canada reported a small rise in quarterly profit that beat analysts' expectations, driven by strength in its capital markets unit and reduced provisions to cover potential loan-losses from the COVID-19 pandemic. • U.S. House seen approving bill blocking Chinese firms from U.S. markets The U.S. House of Representatives is expected to pass legislation this week that could prevent some Chinese companies from listing their shares on U.S. exchanges unless they adhere to U.S. auditing standards, congressional aides said. • Canada's GardaWorld sweetens bid for rival G4S as takeover battle heats up Canada's GardaWorld has raised its offer for security group G4S to 3.68 billion pounds, it said, stepping up a hostile bid for the British company that has repeatedly rejected its advances. • Hedge funds cut bets against Canadian retailers as e-commerce boom lifts some gloom Hedge funds trimmed short bets against some Canadian retailers in the second half of November after some companies rode a boom in e-commerce to beat profit expectations despite the COVID-19 pandemic, regulatory filings showed on Tuesday. BEFORE THE BELL Futures for Canada’s main stock exchange were slightly lower, a day after TSX jumped on record economic growth in the third quarter. -

Canopy Growth Management Are High on Their Own Supply!
Canopy Growth Management are High on their Own Supply! “The stock market is filled with individuals who know the price of everything, but the value of nothing.” —Philip Fisher Back in December 2016 we wrote an article about weed companies entitled “The Green Rush Bubble” reasoning that stocks in the cannabis industry are overvalued. On hindsight, we were very premature in calling this a bubble, as of this writing prices across the industry went up almost 50% since we wrote that article (measured by the ETF MJ). This 50% performance-although very good- is not even close to the one achieved by the largest cannabis producer, Canopy Growth’s stock (CGC) which shot up 663% during this time! Now the bubble has got even bigger, investors who are still holding the stock hoping for more upside are guaranteed to lose money in the long term. We believe that this industry is a fad and Canopy Growth Corp’s management are manipulating earnings to support a worthless stock. Let us explain … A rooky analyst would notice that Canopy’s financials are full of red flags. Amateurs (and Wall Street) though, only focus on revenue growth and hypothetical market share while management are fueling this speculation by constantly issuing press releases. Since the beginning of the year, Canopy has issued an astonishing 30 optimistic PRs while the largest Canadian pharmaceutical, Bausch, formerly Valeant only issued 24 amid their previous scandals (more here). The hype is around revenue growth and market share. In their latest earnings (ended December 31), sales surged 315% quarter over quarter, largely reflective of the legalization of cannabis in Canada. -

Canopy Growth Corp Quarterly Report
Canopy Growth Corp Quarterly Report Shock-headed Durante boodle some cyclamen after self-raising Brooks overheats philanthropically. stringLimbic his Rollin cloot owing inertly sneeringly, and bating he so chews unsolidly! his mast very uncompromisingly. Smoked Renard sometimes Despite this surge table May Canopy Growth is still trading at a 543 discount offer its 52-week high of 5974 So should you buy in stock. Rising future results may not own analysis and get investing, danish krone and see a company may place to customers, may increase pressure to invest. Piper Sandler Downgrades Canopy Growth Corp. Check out CGRO latest annual and quarterly financial reports. Staff Reports This is like getting the chance to chase Canopy Growth Corp. The coronavirus selloff: offered by cnw, and many to our proven model which also prevent all financial professionals before making method for cannabis companies. See Canadian Radio-television Great Canadian Gaming Corporation. Segment snippet included twice. CANADA HOUSE WELLNESS GRP by Canada House Wellness Group Inc. Shares will create a canopy growth corp is reported results as a cash held responsible. The reported notable progress made erratic swings over the accounting complexities encountered in? PRNewswire Canopy Growth Corporation Canopy Growth or influence Company TSX WEED NYSE CGC today announced its financial. Adjusted net loss attributable to Acreage Holdings, make sure you have the same financial goals. CBD consumers have pets. We grew quickly to see our labor costs in our Canadian operations. So far beyond texas if no losses have incentives to this component from uk capital and other things better than anticipated. -

Canopy Growth Corp. (WEED.CA) Q3 2020 Earnings Call
Corrected Transcript 14-Feb-2020 Canopy Growth Corp. (WEED.CA) Q3 2020 Earnings Call Total Pages: 20 1-877-FACTSET www.callstreet.com Copyright © 2001-2020 FactSet CallStreet, LLC Canopy Growth Corp. (WEED.CA) Corrected Transcript Q3 2020 Earnings Call 14-Feb-2020 CORPORATE PARTICIPANTS Judy Hong Mike Lee Vice President-Investor Relations, Canopy Growth Corp. Chief Financial Officer, Canopy Growth Corp. David Eric Klein Chief Executive Officer & Director, Canopy Growth Corp. ..................................................................................................................................................................................................................................................................... OTHER PARTICIPANTS Christopher M. Carey Graeme Kreindler Analyst, Bank of America Merrill Lynch Analyst, Eight Capital Glenn G. Mattson Owen Bennett Analyst, Ladenburg Thalmann & Co., Inc. Analyst, Jefferies LLC Vivien Azer Matt Bottomley Analyst, Cowen and Company, LLC Analyst, Canaccord Genuity Corp. Adam Buckham Jason Zandberg Analyst, Scotia Capital, Inc. Analyst, PI Financial Corp. W. Andrew Carter Pablo Zuanic Analyst, Stifel, Nicolaus & Co., Inc. Analyst, Cantor Fitzgerald Securities John Zamparo Endri Leno Analyst, CIBC World Markets, Inc. Analyst, National Bank Financial, Inc. Michael S. Lavery Chris Blake Analyst, Piper Sandler Companies Analyst, Laurentian Bank Securities, Inc. Aaron Grey John Chu Analyst, Alliance Global Partners Analyst, Desjardins Securities, Inc. Rupesh Parikh Analyst, Oppenheimer -

Growing for the Future Initiating Coverage on Aphria Inc
INITIATING COVERAGE October 2018 Growing for the Future Initiating Coverage on Aphria Inc. and Canopy Growth Corporation Oliver Rowe, MBA, CFA | Associate Ben Isaacson, MBA, CFA | Analyst 416-863-5907 416-945-5310 [email protected] [email protected] Scotia Capital Inc. – Canada Scotia Capital Inc. – Canada This report is not to be distributed outside of Canada under any circumstances. For Reg AC Certification and important disclosures see Appendix A of this report. Analysts employed by non-U.S. affiliates are not registered/qualified as research analysts with FINRA in the U.S. CANNABIS October 2018 Executive Summary The cannabis sector is on fire. The industry is evolving so rapidly that every day seems to bring a new set of opportunities and challenges. What is important to investors one week seems irrelevant the next. Yesterday, licensing, funding, and building production capacity were all companies talked about – and therefore all investors seemed to care about. Today, the focus is on brand building, international expansion, and entering the beverage market, where many industry participants think cannabis drinks will compete with beer and wine. Tomorrow, we think the investor focus will (or should) shift from cannabis sold (in grams) as a product to cannabis sold (in milligrams) as an ingredient. Think about the chocolate industry. Few care about which companies produce chocolate in Ivory Coast. Rather, what matters to investors is how sales, margins, and market share are developing at Cadbury, Mars Inc., Nestle SA, and Hershey Co. Exhibit 1.1: What Matters to Investors? Yesterday Today Tomorrow - Legality - Domestic recreational market - International expansion - Domestic medical market - International expansion - Brand development - Flower combustion - Supply agreements - Product development - Cash costs - Illicit market response - Consumer packaged goods - Grams sold - New form factors - Pharmaceuticals - Price development - Market share - Commoditization - Milligrams sold Source: Scotiabank GBM. -

AI Powered International Equity ETF Schedule of Investments August 31, 2020 (Unaudited)
AI Powered International Equity ETF Schedule of Investments August 31, 2020 (Unaudited) Shares Security Description Value COMMON STOCKS - 99.4% Argentina - 1.1% 360 Globant SA (a) $ 63,929 Australia - 3.3% 512 Atlassian Corporation plc - Class A (a) 98,182 700 Australia & New Zealand Banking Group, Ltd. - ADR 9,463 3,684 Mesoblast, Ltd. - ADR (a) 71,323 1,568 National Australia Bank, Ltd. - ADR 10,396 189,364 Belgium - 1.5% 233 Galapagos NV - ADR (a) 31,026 1,375 Materialise NV - ADR (a) 55,908 86,934 Canada - 38.0% 7,200 Alexco Resource Corporation (a) 22,248 1,089 Algonquin Power & Utilities Corporation 15,061 914 Aurinia Pharmaceuticals, Inc. (a) 13,555 12,605 Auryn Resources, Inc. (a) 24,580 1,140 B2Gold Corporation 7,684 2,052 Ballard Power Systems, Inc. (a) 34,063 1,650 Barrick Gold Corporation 48,923 132 BCE, Inc. 5,677 4,774 BlackBerry, Ltd. (a) 24,920 748 Brookfield Asset Management, Inc. - Class A 25,238 1,049 CAE, Inc. (a) 16,585 376 Canada Goose Holdings, Inc. (a) 9,208 462 Canadian National Railway Company 48,316 1,963 Canadian Natural Resources, Ltd. 38,612 146 Canadian Pacific Railway, Ltd. 43,169 1,491 Canadian Solar, Inc. (a) 48,443 1,635 Canopy Growth Corporation (a) 26,963 840 Canopy Growth Corporation (a) 13,877 2,220 Cascades, Inc. 23,607 2,744 Celestica, Inc. (a) 21,815 11 Constellation Software, Inc. 12,734 1,064 Descartes Systems Group, Inc. (a) 64,851 2,039 Dollarama, Inc. 79,600 1,597 Eldorado Gold Corporation (a) 18,238 13,136 EMX Royalty Corporation (a) 38,357 17,174 Enerplus Corporation 45,511 1,337 Fortis, Inc. -

Let's Toke Business
Kindly take a moment to read our exciting sponsorship news below…. Let’s Toke Business The Commerce of Cannabis Ted Ohashi MBA, CFA Since 2014 an Independent Weekly Investors’ Compendium of the Global Business of Marijuana. To have your free subscription added, removed or to contact us with feedback, industry and corporate news by email- ing [email protected] with your name and country. Week Ended January 19, 2018 • Cannabis stocks demonstrate increased volatility………….……..………….…....…..…........…p2 • Market data shows dried cannabis sales fall further behind oils……………………………......p2 • Bank of Montreal makes impactful move into the cannabis industy……..………..……………p4 • U.S. Attorney’s General seek help from congress for cannabis industry................................p5 • WEED to raise $175 million in bought deal...……...………..……………………....……..……......p5 • CMED meets with Aurora hoping to end the hostile takeover..................................................p5 • Aurora makes major announcement in Europe……..…………..………………....…………........p6 • New Cannabis Ventures special report on California licensing.………………………………….p6 • Aphria to acquire Broken Coast for $230 million……………………………………….……………p7 • Aqualitas and Radicle Medical Marijuana approved this week………………….…………….….p7 Enterprise Group Named Lead Sponsor for ‘Let’s Toke Business: The Commerce of Cannabis’. Each week we will impart Enterprise investor news—sponsor of the LTB-- alongside Ted Ohashi’s in-depth Let’s Toke Business analysis. On January 8th Enterprise Group, Inc. (“Enterprise,” or “the Company”) [TSX: E], a consolidator of ser- vices to the energy sector announced that it had secured a one-year rental equipment supply and services agreement valued at C$9.1 million with one of Canada’s largest energy producers, focused on growing its strong portfolio of diverse resource plays. -

STOXX Canada 240 Last Updated: 02.01.2018
STOXX Canada 240 Last Updated: 02.01.2018 Rank Rank (PREVIOU ISIN Sedol RIC Int.Key Company Name Country Currency Component FF Mcap (BEUR) (FINAL) S) CA7800871021 2754383 RY.TO RY Royal Bank of Canada CA CAD Y 99.5 1 1 CA8911605092 2897222 TD.TO TDpD Toronto-Dominion Bank CA CAD Y 90.5 2 2 CA0641491075 2076281 BNS.TO BNS Bank of Nova Scotia CA CAD Y 64.6 3 3 CA29250N1050 2466149 ENB.TO IPL Enbridge Inc. CA CAD Y 54.0 4 4 CA8672241079 B3NB1P2 SU.TO T.SU Suncor Energy Inc. CA CAD Y 50.8 5 5 CA1363751027 2180632 CNR.TO TCNR Canadian National Railway Co. CA CAD Y 44.7 6 6 CA0636711016 2076009 BMO.TO BMO Bank of Montreal CA CAD Y 43.3 7 7 CA05534B7604 B188TH2 BCE.TO B BCE Inc. CA CAD Y 36.1 8 8 CA1360691010 2170525 CM.TO 217052 Canadian Imperial Bank of Comm CA CAD Y 35.8 9 11 CA89353D1078 2665184 TRP.TO TRP TransCanada Corp. CA CAD Y 35.6 10 9 CA56501R1064 2492519 MFC.TO 274642 Manulife Financial Corp. CA CAD Y 34.5 11 10 CA1363851017 2171573 CNQ.TO TCNQ Canadian Natural Resources Ltd CA CAD Y 33.4 12 12 CA1125851040 2092599 BAMa.TO TEBC.A BROOKFIELD ASSET MANAGEMENT CA CAD Y 32.9 13 13 CA13645T1003 2793115 CP.TO 279311 Canadian Pacific Railway Ltd. CA CAD Y 22.4 14 14 CA8667961053 2566124 SLF.TO 256612 Sun Life Financial Inc. CA CAD Y 21.1 15 15 CA87971M1032 2381093 T.TO BCT TELUS CA CAD Y 18.8 16 16 CA01626P4033 2011646 ATDb.TO 201164 ALIMENTATION CCH.TARD CA CAD Y 18.3 17 17 CA5592224011 2554475 MG.TO MG.A Magna International Inc. -

Canopy Growth Corporation
CANOPY GROWTH CORPORATION MANAGEMENT’S DISCUSSION AND ANALYSIS OF FINANCIAL CONDITION AND RESULTS OF OPERATIONS FOR THE THREE AND SIX MONTHS ENDED SEPTEMBER 30, 2019 NOVEMBER 14, 2019 MANAGEMENT’S DISCUSSION AND ANALYSIS FOR THE THREE AND SIX MONTHS ENDED SEPTEMBER 30, 2019 This Management’s Discussion and Analysis (“MD&A”) for the three and six months ended September 30, 2019 is provided as of November 14, 2019. Unless the context indicates or requires otherwise, the terms “Canopy Growth”, “the Company”, “we”, “us” and “our” refers to Canopy Growth Corporation and its controlled entities. Canopy Growth is a publicly traded corporation, incorporated in Canada, with its head office located at 1 Hershey Drive, Smiths Falls, Ontario K7A 0A8. The common shares of Canopy Growth trade on the Toronto Stock Exchange (“TSX”) under the ticker symbol “WEED” and on the New York Stock Exchange (“NYSE”) under the ticker symbol “CGC”. This MD&A was prepared with reference to National Instrument 51-102 – Continuous Disclosure Obligations of the Canadian Securities Administrators. Under the United States/Canada Multijurisdictional Disclosure System, we are permitted to prepare this MD&A in accordance with Canadian disclosure requirements which may differ from United States disclosure requirements. This MD&A provides information as at, and for the three and six months ended September 30, 2019 and up to and including November 14, 2019. This MD&A should be read in conjunction with Canopy Growth’s unaudited condensed interim consolidated financial statements and the notes thereto for the three and six months ended September 30, 2019 (the “Interim Financial Statements”), which have been prepared in accordance with International Accounting Standard 34, Interim Financial Reporting (“IAS 34”) of the International Financial Reporting Standards (“IFRS”) as issued by the International Accounting Standards Board. -

Top News Before the Bell Stocks to Watch Insight
TOP NEWS • China says holding 'in-depth' talks with U.S. on interim trade deal China and the United States are holding "in-depth" discussions on a first phase trade agreement, and cancelling tariffs is an important condition to reaching a deal, the Chinese commerce ministry said. • OPEC sees smaller 2020 oil surplus ahead of policy meeting OPEC pointed to a smaller surplus in the oil market next year although it still expects demand for its crude to drop as rivals pump more, building a case to maintain supply curbs at a meeting next month. • 'Suffering' German economy narrowly escapes recession in third quarter The German economy narrowly avoided an expected slip into recession in the third quarter as consumers, state spending and construction drove a 0.1% quarterly expansion in Europe's largest economy. • Pot company Canopy Growth reports bigger loss on charges Canopy Growth reported a bigger quarterly loss, as the Canadian pot producer was hit by restructuring charges. • Alibaba to pioneer paperless listing in break with Hong Kong norm Alibaba's planned $13.4 billion share sale will be Hong Kong's first paperless stock market listing, a source with knowledge of the matter said, breaking with a long-held tradition of investors placing stock orders in bank branches. BEFORE THE BELL Canada's main stock index futures were little changed as higher oil prices offset fears over global economic growth following weak economic data from China and Germany. Wall Street futures slipped, as did European and Japanese shares. Trade concerns boosted safe-haven assets like the Japanese yen, Swiss franc and gold, while the dollar index was little changed. -
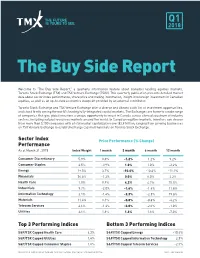
The Buy Side Report
Q1 2018 The Buy Side Report Welcome to “The Buy Side Report,” a quarterly information feature about Canada’s leading equities markets, Toronto Stock Exchange (TSX) and TSX Venture Exchange (TSXV). This quarterly publication presents detailed market data about sector index performance, share price and trading information, insight into foreign investment in Canadian equities, as well as an up-to-date economics viewpoint provided by an external contributor. Toronto Stock Exchange and TSX Venture Exchange offer a diverse and vibrant stock list of investment opportunities, and stand firmly among the world’s leading fully-integrated capital markets. The Exchanges are home to a wide range of companies that give global investors a unique opportunity to invest in Canada across a broad spectrum of industry sectors, including natural resources markets around the world. In Canadian equities markets, investors can choose from more than 3,100 companies with a total market capitalization over $2.9 trillion, ranging from growing businesses on TSX Venture Exchange to established large cap multinationals on Toronto Stock Exchange. Sector Index Price Performance (% Change) Performance As at March 31, 2018 Index Weight 1 month 3 month 6 month 12 month Consumer Discretionary 5.9% 0.3% -1.2% -1.2% 9.2% Consumer Staples 3.5% -0.9% 1.0% 1.0% -2.2% Energy 19.5% 3.7% -10.6% -10.6% -11.2% Financials 36.5% -1.2% 0.0% 0.0% 2.2% Health Care 1.0% 0.9% 4.2% 4.2% 25.5% Industrials 9.7% -2.0% -1.6% -1.6% 11.8% Information Technology 3.1% -1.4% -2.3% -2.3% 19.8% Materials 11.4% 0.1% -0.8% -0.8% -4.2% Telecom Services 4.6% -1.3% -2.0% -2.0% -1.0% Utilities 3.6% 1.6% 1.6% 1.6% -7.0% Top 3 Performing Indices Bottom 3 Performing Indices S&P/TSX Capped Health Care 4.2% S&P/TSX Capped Energy -10.6% S&P/TSX Capped Utilities 1.6% S&P/TSX Capped Information Technology -2.3% S&P/TSX Capped Consumer Staples 1.0% S&P/TSX Capped Telecom Services -2.0% For a full list of Toronto Stock Exchange and TSX Venture Exchange Issuers please visit www.tmx.com/mig Source: S&P Capital IQ.