The Argent and Sable and Other Moths Associated with Bog Myrtle in North Wales
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

LANDFIRE Biophysical Setting Model
LANDFIRE Biophysical Setting Model Biophysical Setting 7316170 Western North American Boreal Shrub and Herbaceous Floodplain Wetland This BPS is lumped with: This BPS is split into multiple models: General Information Contributors (also see the Comments field Date 5/7/2008 Modeler 1 Tina Boucher [email protected] Reviewer Michelle Schuman michelle.schuman@a k.usda.gov Modeler 2 Kori Blankenship [email protected] Reviewer Modeler 3 Colleen Ryan [email protected] Reviewer Vegetation Type Map Zone Model Zone Wetlands/Riparian 73 Alaska N-Cent.Rockies California Pacific Northwest Dominant Species* General Model Sources Great Basin South Central Literature METR3 ERAN6 Great Lakes Southeast Local Data CAAQ MYGA Northeast S. Appalachians EQFL BENA Expert Estimate Northern Plains Southwest COPA28 CHCA2 Geographic Range This system is found throughout the boreal and sub-boreal regions. It occurs within the floodplain boundaries of the Western North American Boreal Montane Floodplain Forest and Shrubland - Boreal, Western North American Boreal Lowland Large River Floodplain Forest and Shrubland and Western North American Boreal Montane Floodplain Forest and Shrubland - Alaska Sub-boreal. Biophysical Site Description The following information was taken from the draft Boreal Ecological Systems description (NatureServe 2008): Floodplain wetlands occur within the active and inactive portions of floodplain systems. Wetlands develop on poorly drained deposits, oxbows and abandoned channels and are often mosaiced with well- drained floodplain vegetation. Vegetation Description Species composition is variable due to diverse environmental conditions such as water depth, substrate and nutrient input, as well as seral stage. Aquatic bed, marsh and fen communities are common. The earliest seral stages (aquatic bed) have aquatic vegetation such as Nuphar polysepala. -

Chemical Composition and Antimicrobial Activity of Fruit Essential Oils of Myrica Gale, a Neglected Non-Wood Forest Product
BALTIC FORESTRY http://www.balticforestry.mi.lt Baltic Forestry 2020 26(1): 13–20 ISSN 1392-1355 Category: research article eISSN 2029-9230 https://doi.org/10.46490/BF423 Chemical composition and antimicrobial activity of fruit essential oils of Myrica gale, a neglected non-wood forest product KRISTINA LOŽIENĖ1,2, JUOZAS LABOKAS1*, VAIDA VAIČIULYTĖ1, JURGITA ŠVEDIENĖ1, VITA RAUDONIENĖ1, ALGIMANTAS PAŠKEVIČIUS1, LAIMA ŠVEISTYTĖ3 AND VIOLETA APŠEGAITĖ1 1 Nature Research Centre, Akademijos g. 2, LT-08412 Vilnius, Lithuania 2 Farmacy Center, Institute of Biomedical Science, Faculty of Medicine, Vilnius University, M.K. Čiurlionio g. 21/27, LT-03101 Vilnius, Lithuania 3 Plant Gene Bank, Stoties g. 2, Akademija, LT-58343 Kėdainių r., Lithuania * Corresponding author: [email protected], phone: +370 5 2729930 Ložienė, K., Labokas, J., Vaičiulytė, V., Švedienė, J., Raudonienė, V., Paškevičius, A., Šveistytė, L. and Apšegaitė, V. 2020. Chemical composition and antimicrobial activity of fruit essential oils of Myrica gale, a neglected non-wood forest product. Baltic Forestry 26(1): 13–20. https://doi.org/10.46490/BF423. Received 18 October 2019 Revised 25 March 2020 Accepted 28 April 2020 Abstract The study aimed to establish the chemical composition of fruit essential oils of M. gale and test their activities against the selected pathogenic bacteria (Staphylococcus aureus, Escherichia coli, Acinetobacter baumannii), yeasts (Candida albicans, C. parapsilosis), fungi (Aspergillus fumigatus, A. flavus) and dermatophytes (Trichophyton rubrum, T. mentagrophytes). Fruit samples from natural (Western Lithuania) and anthropogenic (Eastern Lithuania) M. gale populations were studied separately. Essential oils were isolated from dried fruits by hydrodistillation and analysed by GC/FID and GC/MS methods; enantiomeric composition of α-pinene was established by chiral-phase capillary GC. -

Introduction to Common Native & Invasive Freshwater Plants in Alaska
Introduction to Common Native & Potential Invasive Freshwater Plants in Alaska Cover photographs by (top to bottom, left to right): Tara Chestnut/Hannah E. Anderson, Jamie Fenneman, Vanessa Morgan, Dana Visalli, Jamie Fenneman, Lynda K. Moore and Denny Lassuy. Introduction to Common Native & Potential Invasive Freshwater Plants in Alaska This document is based on An Aquatic Plant Identification Manual for Washington’s Freshwater Plants, which was modified with permission from the Washington State Department of Ecology, by the Center for Lakes and Reservoirs at Portland State University for Alaska Department of Fish and Game US Fish & Wildlife Service - Coastal Program US Fish & Wildlife Service - Aquatic Invasive Species Program December 2009 TABLE OF CONTENTS TABLE OF CONTENTS Acknowledgments ............................................................................ x Introduction Overview ............................................................................. xvi How to Use This Manual .................................................... xvi Categories of Special Interest Imperiled, Rare and Uncommon Aquatic Species ..................... xx Indigenous Peoples Use of Aquatic Plants .............................. xxi Invasive Aquatic Plants Impacts ................................................................................. xxi Vectors ................................................................................. xxii Prevention Tips .................................................... xxii Early Detection and Reporting -

Somerset's Ecological Network
Somerset’s Ecological Network Mapping the components of the ecological network in Somerset 2015 Report This report was produced by Michele Bowe, Eleanor Higginson, Jake Chant and Michelle Osbourn of Somerset Wildlife Trust, and Larry Burrows of Somerset County Council, with the support of Dr Kevin Watts of Forest Research. The BEETLE least-cost network model used to produce Somerset’s Ecological Network was developed by Forest Research (Watts et al, 2010). GIS data and mapping was produced with the support of Somerset Environmental Records Centre and First Ecology Somerset Wildlife Trust 34 Wellington Road Taunton TA1 5AW 01823 652 400 Email: [email protected] somersetwildlife.org Front Cover: Broadleaved woodland ecological network in East Mendip Contents 1. Introduction .................................................................................................................... 1 2. Policy and Legislative Background to Ecological Networks ............................................ 3 Introduction ............................................................................................................... 3 Government White Paper on the Natural Environment .............................................. 3 National Planning Policy Framework ......................................................................... 3 The Habitats and Birds Directives ............................................................................. 4 The Conservation of Habitats and Species Regulations 2010 .................................. -

Chapter 5: Vegetation of Sphagnum-Dominated Peatlands
CHAPTER 5: VEGETATION OF SPHAGNUM-DOMINATED PEATLANDS As discussed in the previous chapters, peatland ecosystems have unique chemical, physical, and biological properties that have given rise to equally unique plant communities. As indicated in Chapter 1, extensive literature exists on the classification, description, and ecology of peatland ecosystems in Europe, the northeastern United States, Canada, and the Rocky Mountains. In addition to the references cited in Chapter 1, there is some other relatively recent literature on peatlands (Verhoeven 1992; Heinselman 1963, 1970; Chadde et al., 1998). Except for efforts on the classification and ecology of peatlands in British Columbia by the National Wetlands Working Group (1988), the Burns Bog Ecosystem Review (Hebda et al. 2000), and the preliminary classification of native, low elevation, freshwater vegetation in western Washington (Kunze 1994), scant information exists on peatlands within the more temperate lowland or maritime climates of the Pacific Northwest (Oregon, Washington, and British Columbia). 5.1 Introduction There are a number of classification schemes and many different peatland types, but most use vegetation in addition to hydrology, chemistry and topological characteristics to differentiate among peatlands. The subject of this report are acidic peatlands that support acidophilic (acid-loving) and xerophytic vegetation, such as Sphagnum mosses and ericaceous shrubs. Ecosystems in Washington state appear to represent a mosaic of vegetation communities at various stages of succession and are herein referred to collectively as Sphagnum-dominated peatlands. Although there has been some recognition of the unique ecological and societal values of peatlands in Washington, a statewide classification scheme has not been formally adopted or widely recognized in the scientific community. -

Lepidoptera: Tortricidae: Tortricinae) and Evolutionary Correlates of Novel Secondary Sexual Structures
Zootaxa 3729 (1): 001–062 ISSN 1175-5326 (print edition) www.mapress.com/zootaxa/ Monograph ZOOTAXA Copyright © 2013 Magnolia Press ISSN 1175-5334 (online edition) http://dx.doi.org/10.11646/zootaxa.3729.1.1 http://zoobank.org/urn:lsid:zoobank.org:pub:CA0C1355-FF3E-4C67-8F48-544B2166AF2A ZOOTAXA 3729 Phylogeny of the tribe Archipini (Lepidoptera: Tortricidae: Tortricinae) and evolutionary correlates of novel secondary sexual structures JASON J. DOMBROSKIE1,2,3 & FELIX A. H. SPERLING2 1Cornell University, Comstock Hall, Department of Entomology, Ithaca, NY, USA, 14853-2601. E-mail: [email protected] 2Department of Biological Sciences, University of Alberta, Edmonton, Canada, T6G 2E9 3Corresponding author Magnolia Press Auckland, New Zealand Accepted by J. Brown: 2 Sept. 2013; published: 25 Oct. 2013 Licensed under a Creative Commons Attribution License http://creativecommons.org/licenses/by/3.0 JASON J. DOMBROSKIE & FELIX A. H. SPERLING Phylogeny of the tribe Archipini (Lepidoptera: Tortricidae: Tortricinae) and evolutionary correlates of novel secondary sexual structures (Zootaxa 3729) 62 pp.; 30 cm. 25 Oct. 2013 ISBN 978-1-77557-288-6 (paperback) ISBN 978-1-77557-289-3 (Online edition) FIRST PUBLISHED IN 2013 BY Magnolia Press P.O. Box 41-383 Auckland 1346 New Zealand e-mail: [email protected] http://www.mapress.com/zootaxa/ © 2013 Magnolia Press 2 · Zootaxa 3729 (1) © 2013 Magnolia Press DOMBROSKIE & SPERLING Table of contents Abstract . 3 Material and methods . 6 Results . 18 Discussion . 23 Conclusions . 33 Acknowledgements . 33 Literature cited . 34 APPENDIX 1. 38 APPENDIX 2. 44 Additional References for Appendices 1 & 2 . 49 APPENDIX 3. 51 APPENDIX 4. 52 APPENDIX 5. -

Antimicrobial Properties of Traditional Brewing Herbs
ANTIMICROBIAL PROPERTIES OF TRADITIONAL BREWING HERBS - Ledum palustre, Myrica gale & Humulus lupulus Gordon Virgo Project work in Ethnobiology 15 hp, 2010 Evolutionary Biology Centre (EBC) Supervisor: Hugo J. de Boer ACKNOWLEDGEMENTS I would like to thank my supervisor Hugo de Boer for encouragement and his excellent input to this project. Thanks to Stallhagen Brewery and Marcus Lindborg for providing material and literature. Also, I would like to thank Stefan Roos at SLU and Marianne Svarvare for guidance and technique. ABSTRACT In this study, three different brewing herbs that have been used through the history are evaluated as inhibitors of common beer spoilage organisms. The three species are Ledum palustre (Marsh Tea), Myrica gale (Bog Myrtle) and Humulus lupulus (Hops). Experimental batches of 10 L were made with all the three herbs and one without any additives. For each herb, two batches were made with different concentration, one batch with 3g/L and the other with 6g/L. All batches were treated the same and fermentation pattern for all of them were similar. Inoculations of four common beer spoilage organisms were practiced in order to examine microbial resistance of the different beers. Antibacterial activity was analyzed by membrane filtration and by measure the optical density during the incubation time. Both Humulus lupulus and Myrica gale showed clear resistance to the three gram-positive bacteria. 1 INTRODUCTION Throughout history, several different plant species have been used as beer additives for flavour and above all as preservatives. Particularly two species, Myrica gale and Humulus lupulus were widely used as beer additives in Europe (Behre 1999). -

Lockerbie Wildlife Trust Eskrigg Reserve May 2016 News Bulletin
Lockerbie Wildlife Trust (www.lockerbie-wildlife-trust.co.uk) Eskrigg Reserve Scottish Charity No: May 2016 News Bulletin SC 005538 1. Views of the pond on four different days in May. 2. Confirmed wildlife sightings at the Reserve in May. a. Birds Blackbird, Blue Tit, Bullfinch, Buzzard, Carrion Crow, Chaffinch, Chiffchaff, Coal Tit, Cuckoo, Goldcrest, Goldfinch, Goosander, Great Spotted Woodpecker, Great Tit, Greenfinch, Grey Heron, Greylag Goose, Grey Wagtail, House Martin, House Sparrow, Jackdaw, Jay, Kestrel, Lesser Redpoll, Little Grebe, Mallard, Mandarin, Moorhen, Mute Swan, Nuthatch, Oystercatcher, Pheasant, Raven, Robin, Siskin, Song Thrush, Sparrowhawk, Starling, Swallow, Willow Warbler, Wood Pigeon, Wren. b. Mammals Common Shrew, Soprano Pipistrelle Bat, Bank Vole, Mole, Noctule Bat, Rabbit, Red Squirrel, Roe Deer, Wood Mouse. c. Amphibia Frog, Toad. d. Invertebrates Black Slug, Buff-tailed Bumble Bee, Green-veined White, Orange-tip, Small Tortoiseshell Butterflies, Blue-tailed, Common Blue and Large Red Damselflies, Hoverflies, Midges, Brimstone, Nettle Tap and Water Carpet Moths, Drinker Moth caterpillar, Mosquitoes. Photographs: Jim Rae 1" " 3. May Photo-gallery. Row 1: Female Mallard and ducklings (JR), Robin (JR), Little Grebe (JR) Row 2: Young Red Squirrel (JR), Cuckoo Flower (JR), Bank Vole (BL) Row 3: Grey Heron (JR), Pink Purslane / Common Stork's-bill (JR), Great Spotted Woodpecker (JR) Row 4: Spider (JR), Nettle-tap (JR), Non-biting Midge (JR) Photographs: Bob Little (BL), Jim Rae (JR) 2" " 4. Planned Activities 4th/5th - Jim attended a Small Mammal Survey and Identification course run by Andy Riches, Dumfries and Galloway Mammal Recorder, at Rouken Glen Country Park. 12th - Jim attended a Squirrel Hair Training course run by Alexa Seagrave, Saving Scotland's Red Squirrels, at Lochfield Cottage, Lochmaben. -

Seed Dispersal and Propagation of Three Myrica Species
Of Birds and Bayberries : Alfred j. Fordham Seed Dispersal and Propagation of Three Myrica Species The genus Myrica comprises about 50 tral United States and from Europe to north- species (often ill-defined) distributed east Asia. throughout the temperate and subtropical All three of these species have nitrogen- areas of both hemispheres. The Arnold Ar- fixing root nodules, which enable them to boretum collection includes three species: thrive m areas where many other plants M. pensylvanica, M. cerifera, andM. gale. could not survive. They are dioecious - Myrica pensylvanica Lois., the common having staminate (male) and pistillate (fe- bayberry or candleberry, occurs naturally male) flowers on different plants - like hol- from Newfoundland to western New York lies and ashes. and Maryland, chiefly in poor soil. It is suck- The fruits of Myrica pensylvanica and M. ering in habit and tends to form shrubby cerifera are small (2.5-3 mm and 3.5~.5 mm clumps, which at maturity can range from 2 in diameter respectively) globose nuts with to 8 feet in height. Frequently it is found on waxlike coatings. It is this waxlike material roadside cuts, railroad banks, gravel pits, and that provides the fragrance in bayberry- other locations where topsoil has been re- scented candles and soap. It becomes bluish moved completely. In Boston its shiny green gray as it dries, making the thickly clustered leaves remain on the branches until No- fruits conspicuous in the landscape. The vember. They are fragrant when crushed, a fruits ripen in late September and are eaten characteristic of all Myrica species. -

The Changing Ground Flora of an Upland Planted Woodland
The changing ground flora of an upland planted woodland John Holland, SRUC Hill & Mountain Research Centre, Kirkton & Auchtertyre Farms SRUC Hill & Mountain Research Centre Loch Lomond & the Trossachs National Park 2 Caol Gleann Gleann a’Chlachain 3 Gleann a’Chlachain Main Woodland (217 ha) 4 Allt Gleann a’Chlachain Gorge Woodland (43 ha) 5 Trees planted in the in the Allt Gleann a‘Chlachain Gorge Woodland and main Gleann a‘Chlachain Woodland (260 ha) Latin Name English Name Total Percentage Betula pubescens Downy Birch 219710 45.35% Betula pendula Silver Birch 63505 13.11% Pinus sylvestris Scot’s Pine 49300 10.18% Alnus glutinosa Common Alder 43765 9.03% Sorbus aucuparia Rowan 38110 7.87% Salix cinerea Grey Willow 23250 4.80% Salix caprea Goat Willow 17505 3.61% Quercus petraea Sessile Oak 8850 1.83% Salix aurita Eared Willow 6250 1.29% Salix mysinifolia Dark-leaved Willow 5010 1.03% Fraxinus excelsior Ash 4060 0.84% Corylus avellana Hazel 3300 0.68% Populus tremula Aspen 1110 0.23% Salix lapponum Downy Willow 750 0.15% Total 484,475 100% 6 7 8 9 10 11 12 Downy Willow (Salix lapponum) 13 14 Vegetation Changes in the Gorge Woodland • Increase in dwarf shrubs, particularly Ling (Calluna vulgaris) • Increase in Bog Myrtle (Myrica gale) 15 Bog Myrtle (Myrica gale) 16 Vegetation Changes in the Gorge Woodland • Increase in dwarf shrubs, particularly Ling (Calluna vulgaris) • Increase in Bog Myrtle (Myrica gale) • Increase in Purple Moor Grass (Molinia caerulea) and a decrease in Mat Grass (Nardus stricta) 17 Scotch Argus (Erebia aethiops) -

Toby Austin's Garden Moth List
Toby Austin’s Garden Moth List Orange Swift Triodia sylvina Common Swift Korscheltellus lupulinus Ghost Moth Hepialus humuli Nematopogon swammerdamella Cork Moth Nemapogon cloacella Tinea trinotella Horse-chestnut Leaf-miner Cameraria ohridella Bird-cherry Ermine Yponomeuta evonymella Orchard/Apple/Spindle Ermine Yponomeuta padella/malinellus/cagnagella Willow Ermine Yponomeuta rorrella Yponomeuta plumbella Ypsolopha ustella Diamond-back Moth Plutella xylostella Plutella porrectella Ash Bud Moth Prays fraxinella Hawthorn Moth Scythropia crataegella Oegoconia quadripuncta/caradjai/deauratella Crassa unitella Carcina quercana Luquetia lobella Agonopterix alstromeriana Mompha sturnipennella Blastobasis adustella Blastobasis lacticolella Twenty-plume Moth Alucita hexadactyla Beautiful Plume Amblyptilia acanthadactyla Stenoptilia pterodactyla Stenoptilia bipunctidactyla Common Plume Emmelina monodactyla Variegated Golden Tortrix Archips xylosteana Argyrotaenia ljungiana Chequered Fruit-tree Tortrix Pandemis corylana Dark Fruit-tree Tortrix Pandemis heparana Pandemis dumetana Syndemis musculana Lozotaenia forsterana Carnation Tortrix Cacoecimorpha pronubana Light Brown Apple Moth Epiphyas postvittana Lozotaeniodes formosana Summer Fruit Tortrix Adoxophyes orana Flax Tortrix Cnephasia asseclana Green Oak Tortrix Tortrix viridana Cochylis molliculana Cochylis atricapitana Acleris holmiana Acleris forsskaleana Acleris comariana/laterana Acleris cristana Garden Rose Tortrix Acleris variegana Pseudargyrotoza conwagana Phtheochroa rugosana Agapeta -
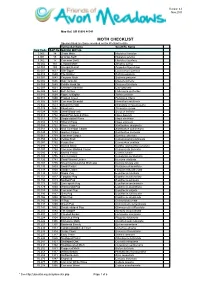
MOTH CHECKLIST Species Listed Are Those Recorded on the Wetland to Date
Version 4.0 Nov 2015 Map Ref: SO 95086 46541 MOTH CHECKLIST Species listed are those recorded on the Wetland to date. Vernacular Name Scientific Name New Code B&F No. MACRO MOTHS 3.005 14 Ghost Moth Hepialus humulae 3.001 15 Orange Swift Hepialus sylvina 3.002 17 Common Swift Hepialus lupulinus 50.002 161 Leopard Moth Zeuzera pyrina 54.008 169 Six-spot Burnet Zygaeba filipendulae 66.007 1637 Oak Eggar Lasiocampa quercus 66.010 1640 The Drinker Euthrix potatoria 68.001 1643 Emperor Moth Saturnia pavonia 65.002 1646 Oak Hook-tip Drepana binaria 65.005 1648 Pebble Hook-tip Drepana falcataria 65.007 1651 Chinese Character Cilix glaucata 65.009 1653 Buff Arches Habrosyne pyritoides 65.010 1654 Figure of Eighty Tethia ocularis 65.015 1660 Frosted Green Polyploca ridens 70.305 1669 Common Emerald Hermithea aestivaria 70.302 1673 Small Emerald Hemistola chrysoprasaria 70.029 1682 Blood-vein Timandra comae 70.024 1690 Small Blood-vein Scopula imitaria 70.013 1702 Small Fan-footed Wave Idaea biselata 70.011 1708 Single-dotted Wave Idaea dimidiata 70.016 1713 Riband Wave Idaea aversata 70.053 1722 Flame Carpet Xanthorhoe designata 70.051 1724 Red Twin-spot Carpet Xanthorhoe spadicearia 70.049 1728 Garden Carpet Xanthorhoe fluctuata 70.061 1738 Common Carpet Epirrhoe alternata 70.059 1742 Yellow Shell Camptogramma bilineata 70.087 1752 Purple Bar Cosmorhoe ocellata 70.093 1758 Barred Straw Eulithis (Gandaritis) pyraliata 70.097 1764 Common Marbled Carpet Chloroclysta truncata 70.085 1765 Barred Yellow Cidaria fulvata 70.100 1776 Green Carpet Colostygia pectinataria 70.126 1781 Small Waved Umber Horisme vitalbata 70.107 1795 November/Autumnal Moth agg Epirrita dilutata agg.