The Actin-Myosin Interaction in Muscle: Background and Overview
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

The Wiskott-Aldrich Syndrome: the Actin Cytoskeleton and Immune Cell Function
Disease Markers 29 (2010) 157–175 157 DOI 10.3233/DMA-2010-0735 IOS Press The Wiskott-Aldrich syndrome: The actin cytoskeleton and immune cell function Michael P. Blundella, Austen Wortha,b, Gerben Boumaa and Adrian J. Thrashera,b,∗ aMolecular Immunology Unit, UCL Institute of Child Health, London, UK bDepartment of Immunology, Great Ormond Street Hospital NHS Trust, Great Ormond Street, London, UK Abstract. Wiskott-Aldrich syndrome (WAS) is a rare X-linked recessive primary immunodeficiency characterised by immune dysregulation, microthrombocytopaenia, eczema and lymphoid malignancies. Mutations in the WAS gene can lead to distinct syndrome variations which largely, although not exclusively, depend upon the mutation. Premature termination and deletions abrogate Wiskott-Aldrich syndrome protein (WASp) expression and lead to severe disease (WAS). Missense mutations usually result in reduced protein expression and the phenotypically milder X-linked thrombocytopenia (XLT) or attenuated WAS [1–3]. More recently however novel activating mutations have been described that give rise to X-linked neutropenia (XLN), a third syndrome defined by neutropenia with variable myelodysplasia [4–6]. WASP is key in transducing signals from the cell surface to the actin cytoskeleton, and a lack of WASp results in cytoskeletal defects that compromise multiple aspects of normal cellular activity including proliferation, phagocytosis, immune synapse formation, adhesion and directed migration. Keywords: Wiskott-Aldrich syndrome, actin polymerization, lymphocytes, -

The Myosin Interacting-Heads Motif Present in Live Tarantula Muscle Explains Tetanic and Posttetanic INAUGURAL ARTICLE Phosphorylation Mechanisms
The myosin interacting-heads motif present in live tarantula muscle explains tetanic and posttetanic INAUGURAL ARTICLE phosphorylation mechanisms Raúl Padróna,1,2, Weikang Mab,1, Sebastian Duno-Mirandac,3, Natalia Koubassovad, Kyoung Hwan Leea,4, Antonio Pintoc, Lorenzo Alamoc, Pura Bolañose, Andrey Tsaturyand, Thomas Irvingb, and Roger Craiga aDivision of Cell Biology and Imaging, Department of Radiology, University of Massachusetts Medical School, Worcester, MA 01655; bBiophysics Collaborative Access Team, Department of Biological Sciences, Illinois Institute of Technology, Chicago, IL 60616; cCentro de Biología Estructural, Instituto Venezolano de Investigaciones Científicas, Caracas 1020A, Venezuela; dInstitute of Mechanics, Moscow State University, 119992 Moscow, Russia; and eCentro de Biofísica y Bioquímica, Instituto Venezolano de Investigaciones Científicas, Caracas 1020A, Venezuela This contribution is part of the special series of Inaugural Articles by members of the National Academy of Sciences elected in 2018. Contributed by Raúl Padrón, April 7, 2020 (sent for review December 6, 2019; reviewed by H. Lee Sweeney and Rene Vandenboom) Striated muscle contraction involves sliding of actin thin filaments frozen-hydrated tarantula thick filaments showed that the helices along myosin thick filaments, controlled by calcium through thin of heads on the filaments were formed by a two-headed assem- filament activation. In relaxed muscle, the two heads of myosin blage that we called the myosin interacting-heads motif (IHM) interact with each other on the filament surface to form the (8, 9), formed by the interaction of a blocked head (BH) and a interacting-heads motif (IHM). A key question is how both heads free head (FH) (10) with the subfragment-2 (8) (Fig. -

Characterization of Cecal Smooth Muscle Contraction in Laying Hens
veterinary sciences Communication Characterization of Cecal Smooth Muscle Contraction in Laying Hens Katrin Röhm 1, Martin Diener 2 , Korinna Huber 1 and Jana Seifert 1,* 1 Institute of Animal Science, University of Hohenheim, 70593 Stuttgart, Germany; [email protected] (K.R.); [email protected] (K.H.) 2 Institute of Veterinary Physiology and Biochemistry, Justus-Liebig University, 35392 Gießen, Germany; [email protected] * Correspondence: [email protected] Abstract: The ceca play an important role in the physiology of the gastrointestinal tract in chickens. Nevertheless, there is a gap of knowledge regarding the functionality of the ceca in poultry, especially with respect to physiological cecal smooth muscle contraction. The aim of the current study is the ex vivo characterization of cecal smooth muscle contraction in laying hens. Muscle strips of circular cecal smooth muscle from eleven hens are prepared to investigate their contraction ex vivo. Contraction is detected using an isometric force transducer, determining its frequency, height and intensity. Spontaneous contraction of the chicken cecal smooth muscle and the influence of buffers (calcium-free buffer and potassium-enriched buffer) and drugs (carbachol, nitroprusside, isoprenaline and Verapamil) affecting smooth muscle contraction at different levels are characterized. A decrease in smooth muscle contraction is observed when a calcium-free buffer is used. Carbachol causes an increase in smooth muscle contraction, whereas atropine inhibits contraction. Nitroprusside, isoprenaline and Verapamil result in a depression of smooth muscle contraction. In conclusion, the present results confirm a similar contraction behavior of cecal smooth muscles in laying hens as Citation: Röhm, K.; Diener, M.; shown previously in other species. -

The Muscular System
THE MUSCULAR SYSTEM COMPILED BY HOWIE BAUM 1 Muscles make up the bulk of the body and account for 1/3 of its weight.!! Blood vessels and nerves run to every muscle, helping control and regulate each muscle’s function. The muscular system creates body heat and also moves the: Bones of the Skeletal system Food through Digestive system Blood through the Circulatory system Fluids through the Excretory system MUSCLE TISSUE The body has 3 main types of muscle tissue 1) Skeletal, 2) Smooth, and 3) Cardiac SKELETAL MUSCLE SMOOTH MUSCLE CARDIAC MUSCLE Skeletal muscles attach to and move bones by contracting and relaxing in response to voluntary messages from the nervous system. Skeletal muscle tissue is composed of long cells called muscle fibers that have a striated appearance. Muscle fibers are organized into bundles supplied by blood vessels and innervated by motor neurons. Muscle structure Skeletal (striated or voluntary) muscle consists of densely packed groups of hugely elongated cells known as myofibers. These are grouped into bundles (fascicles). A typical myofiber is 2–3 centimeters ( 3/4–1 1/5 in) long and 0.05millimeters (1/500 inch) in diameter and is composed of narrower structures – myofibrils. These contain thick and thin myofilaments made up mainly of the proteins actin and myosin. Numerous capillaries keep the muscle supplied with the oxygen and glucose needed to fuel contraction. Skeletal Muscles • Skeletal muscles attach to bones by tendons (connective tissue) and enable movement. • Skeletal muscles are mostly voluntary Feel the back of your ankle to feel your Achilles tendon - the largest tendon in your body. -

Passive Tension in Cardiac Muscle: Contribution of Collagen, Titin, Microtubules, and Intermediate Filaments
Biophysical Journal Volume 68 March 1995 1027-1044 1027 Passive Tension in Cardiac Muscle: Contribution of Collagen, Titin, Microtubules, and Intermediate Filaments Henk L. Granzier and Thomas C. Irving Department of Veterinary and Comparative Anatomy, Pharmacology, and Physiology, Washington State University, Pullman, Washington 99164·6520 USA ABSTRACT The passive tension-sarcomere length relation of rat cardiac muscle was investigated by studying passive (or not activated) single myocytes and trabeculae. The contribution ofcollagen, titin, microtubules, and intermediate filaments to tension and stiffness was investigated by measuring (1) the effects of KCI/KI extraction on both trabeculae and single myocytes, (2) the effect of trypsin digestion on single myocytes, and (3) the effect of colchicine on single myocytes. It was found that over the working range of sarcomeres in the heart (lengths -1.9-2.2 11m), collagen and titin are the most important contributors to passive tension with titin dominating at the shorter end of the working range and collagen at longer lengths. Microtubules made a modest contribution to passive tension in some cells, but on average their contribution was not significant. Finally, intermediate filaments contribl,!ted about 10%to passive tension oftrabeculae at sarcomere lengths from -1.9to 2.1 11m, and theircontribution dropped to only a few percent at longer lengths. At physiological sarcomere lengths of the heart, cardiac titin developed much higher tensions (>20-fold) than did skeletal muscle titin at comparable lengths. This might be related to the finding that cardiac titin has a molecular mass of 2.5 MDa, 0.3-0.5 MDa smaller than titin of mammalian skeletal muscle, which is predicted to result in a much shorter extensible titin segment in the I-band of cardiac muscle. -

Appropriate Roles of Cardiac Troponins in Evaluating Patients with Chest Pain
J Am Board Fam Pract: first published as 10.3122/jabfm.12.3.214 on 1 May 1999. Downloaded from MEDICAL PRACTICE Appropriate Roles of Cardiac Troponins in Evaluating Patients With Chest Pain Matthew S. Rice, MD, CPT, Me, USA, and David C. MacDonald, DO, Me, USA Background: Diagnosis of acute myocardial infarction relies upon the clinical history, interpretation of the electrocardiogram, and measurement of serum levels of cardiac enzymes. Newer biochemical markers of myocardial injury, such as cardiac troponin I and cardiac troponin T, are now being used instead of or along with the standard markers, the MB isoenzyme of creatine kinase (CK-MB) and lactate dehydrogenase. Methods: We performed a MEDLINE literature search (1987 to 1997) using the key words "troponin I," "troponin T," and "acute myocardial infarction." We reviewed selected articles related to the diagnostic and prognostic usefulness of these cardiac markers in evaluating patients with suspected myocardial infarction. Results: We found that (1) troponin I is a better cardiac marker than CK-MB for myocardial infarction because it is equally sensitive yet more specific for myocardial injury; (2) troponin T is a relatively poorer cardiac marker than CK-MB because it is less sensitive and less specific for myocardial injury; and (3) both troponin I and troponin T may be used as independent prognosticators of future cardiac events. Conclusions: Troponin I is a sensitive and specific marker for myocardial injury and can be used to predict the likelihood of future cardiac events. It is not much more expensive to measure than CK-MB. Over all, troponin I is a better cardiac marker than CK-MB and should become the preferred cardiac enzyme when evaluating patients with suspected myocardial infarction. -

Familial Adenomatous Polyposis Polymnia Galiatsatos, M.D., F.R.C.P.(C),1 and William D
American Journal of Gastroenterology ISSN 0002-9270 C 2006 by Am. Coll. of Gastroenterology doi: 10.1111/j.1572-0241.2006.00375.x Published by Blackwell Publishing CME Familial Adenomatous Polyposis Polymnia Galiatsatos, M.D., F.R.C.P.(C),1 and William D. Foulkes, M.B., Ph.D.2 1Division of Gastroenterology, Department of Medicine, The Sir Mortimer B. Davis Jewish General Hospital, McGill University, Montreal, Quebec, Canada, and 2Program in Cancer Genetics, Departments of Oncology and Human Genetics, McGill University, Montreal, Quebec, Canada Familial adenomatous polyposis (FAP) is an autosomal-dominant colorectal cancer syndrome, caused by a germline mutation in the adenomatous polyposis coli (APC) gene, on chromosome 5q21. It is characterized by hundreds of adenomatous colorectal polyps, with an almost inevitable progression to colorectal cancer at an average age of 35 to 40 yr. Associated features include upper gastrointestinal tract polyps, congenital hypertrophy of the retinal pigment epithelium, desmoid tumors, and other extracolonic malignancies. Gardner syndrome is more of a historical subdivision of FAP, characterized by osteomas, dental anomalies, epidermal cysts, and soft tissue tumors. Other specified variants include Turcot syndrome (associated with central nervous system malignancies) and hereditary desmoid disease. Several genotype–phenotype correlations have been observed. Attenuated FAP is a phenotypically distinct entity, presenting with fewer than 100 adenomas. Multiple colorectal adenomas can also be caused by mutations in the human MutY homologue (MYH) gene, in an autosomal recessive condition referred to as MYH associated polyposis (MAP). Endoscopic screening of FAP probands and relatives is advocated as early as the ages of 10–12 yr, with the objective of reducing the occurrence of colorectal cancer. -

ATP and Cellular Work | Principles of Biology from Nature Education
contents Principles of Biology 23 ATP and Cellular Work ATP provides the energy that powers cells. Magnetic resonance images of three different areas in the rat brain show blood flow and the biochemical measurements of ATP, pH, and glucose, which are all measures of energy use and production in brain tissue. The image is color-coded to show spatial differences in the concentration of these energy-related variables in brain tissue. © 1997 Nature Publishing Group Hoehn-Berlage, M., et al. Inhibition of nonselective cation channels reduces focal ischemic injury of rat brain. Journal of Cerebral Blood Flow and Metabolism 17, 534–542 (1997) doi: 10.1097/00004647-199705000-00007. Used with permission. Topics Covered in this Module Using Energy Resources For Work ATP-Driven Work Major Objectives of this Module Describe the role of ATP in energy-coupling reactions. Explain how ATP hydrolysis performs cellular work. Recognize chemical reactions that require ATP hydrolysis. page 116 of 989 4 pages left in this module contents Principles of Biology 23 ATP and Cellular Work Energy is a fundamental necessity for all of life's processes. Without energy, flagella cannot move, DNA cannot be unwound or separated for replication or gene expression, cells cannot divide, plants cannot grow and animals cannot reproduce. Energy is vital, but where does it come from? Plants and photosynthetic microbes capture light energy and convert it into chemical energy for their own use. Organisms that cannot produce their own food, such as fungi and animals, feed upon this captured energy. However, the chemical energy produced by photosynthesizers needs to be converted into a usable form. -

Back-To-Basics: the Intricacies of Muscle Contraction
Back-to- MIOTA Basics: The CONFERENCE OCTOBER 11, Intricacies 2019 CHERI RAMIREZ, MS, of Muscle OTRL Contraction OBJECTIVES: 1.Review the anatomical structure of a skeletal muscle. 2.Review and understand the process and relationship between skeletal muscle contraction with the vital components of the nervous system, endocrine system, and skeletal system. 3.Review the basic similarities and differences between skeletal muscle tissue, smooth muscle tissue, and cardiac muscle tissue. 4.Review the names, locations, origins, and insertions of the skeletal muscles found in the human body. 5.Apply the information learned to enhance clinical practice and understanding of the intricacies and complexity of the skeletal muscle system. 6.Apply the information learned to further educate clients on the importance of skeletal muscle movement, posture, and coordination in the process of rehabilitation, healing, and functional return. 1. Epithelial Four Basic Tissue Categories 2. Muscle 3. Nervous 4. Connective A. Loose Connective B. Bone C. Cartilage D. Blood Introduction There are 3 types of muscle tissue in the muscular system: . Skeletal muscle: Attached to bones of skeleton. Voluntary. Striated. Tubular shape. Cardiac muscle: Makes up most of the wall of the heart. Involuntary. Striated with intercalated discs. Branched shape. Smooth muscle: Found in walls of internal organs and walls of vascular system. Involuntary. Non-striated. Spindle shape. 4 Structure of a Skeletal Muscle Skeletal Muscles: Skeletal muscles are composed of: • Skeletal muscle tissue • Nervous tissue • Blood • Connective tissues 5 Connective Tissue Coverings Connective tissue coverings over skeletal muscles: .Fascia .Tendons .Aponeuroses 6 Fascia: Definition: Layers of dense connective tissue that separates muscle from adjacent muscles, by surrounding each muscle belly. -

GLOSSARY of MEDICAL and ANATOMICAL TERMS
GLOSSARY of MEDICAL and ANATOMICAL TERMS Abbreviations: • A. Arabic • abb. = abbreviation • c. circa = about • F. French • adj. adjective • G. Greek • Ge. German • cf. compare • L. Latin • dim. = diminutive • OF. Old French • ( ) plural form in brackets A-band abb. of anisotropic band G. anisos = unequal + tropos = turning; meaning having not equal properties in every direction; transverse bands in living skeletal muscle which rotate the plane of polarised light, cf. I-band. Abbé, Ernst. 1840-1905. German physicist; mathematical analysis of optics as a basis for constructing better microscopes; devised oil immersion lens; Abbé condenser. absorption L. absorbere = to suck up. acervulus L. = sand, gritty; brain sand (cf. psammoma body). acetylcholine an ester of choline found in many tissue, synapses & neuromuscular junctions, where it is a neural transmitter. acetylcholinesterase enzyme at motor end-plate responsible for rapid destruction of acetylcholine, a neurotransmitter. acidophilic adj. L. acidus = sour + G. philein = to love; affinity for an acidic dye, such as eosin staining cytoplasmic proteins. acinus (-i) L. = a juicy berry, a grape; applied to small, rounded terminal secretory units of compound exocrine glands that have a small lumen (adj. acinar). acrosome G. akron = extremity + soma = body; head of spermatozoon. actin polymer protein filament found in the intracellular cytoskeleton, particularly in the thin (I-) bands of striated muscle. adenohypophysis G. ade = an acorn + hypophyses = an undergrowth; anterior lobe of hypophysis (cf. pituitary). adenoid G. " + -oeides = in form of; in the form of a gland, glandular; the pharyngeal tonsil. adipocyte L. adeps = fat (of an animal) + G. kytos = a container; cells responsible for storage and metabolism of lipids, found in white fat and brown fat. -

Alpha-Actinin-3 R577X
Annals of Applied Sport Science, vol. 4, no. 4, pp. 01-06, Winter 2016 DOI: 10.18869/acadpub.aassjournal.4.4.1 Short Communication www.aassjournal.com www.AESAsport.com ISSN (Online): 2322 – 4479 Received: 20/03/2016 ISSN (Print): 2476–4981 Accepted: 10/06/2016 Alpha-actinin-3 R577X Polymorphism Profile of Turkish Professional Hip-Hop and Latin Dancers 1,2 * 1 1 2 1 1 Korkut Ulucan , Betul Biyik, Sezgin Kapici, Canan Sercan, Oznur Yilmaz, Tunc Catal 1Üsküdar Univerity, Haluk Turksoy Sok. No:14, Altunizade, Üsküdar, İstanbul, Turkey. 2Marmara University, BAsibuyuk Yolu 9/3 MAltepe Saglık Yerleşkesi, MAltepe, Istanbul, Turkey. ABSTRACT Actins are small globular filaments functioning in cell processes like muscle contraction, and stabilized to the sarcomeric Z- discs by actin binding proteins (actinins). One of the important gene coding for actin binding proteins in fast twitch fibers is alpha- actinin- 3 (ACTN3). In this research, we have conducted a gene profile study investigating the genotype and allele distributions of ACTN3 R577X polymorphism in Turkish professional hip- hop and latin dancers and compared them to non-dancers as a control group. 30 professional dancers and non-dancers were recruited for the study. A genotyping procedure was carried out by a newly introduced four-primer PCR methodology. For statistical analysis, the Chi-square test was used to compare data between the groups (p<0,05 evaluated as significant). Numbers and the percentages of dancers were 2 (7%), 21 (70%) and 7(23%) for RR, RX and XX genotypes, respectively. The same numbers and the percentages were 15 (50%), 8 (15%) and 7 (23%) for RR, RX and XX genotypes, respectively, for the controls. -
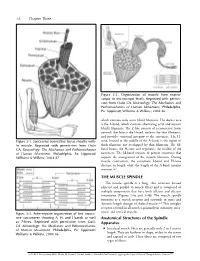
THE MUSCLE SPINDLE Anatomical Structures of the Spindle Apparatus
56 Chapter Three Figure 3-2. Organization of muscle from macro- scopic to microscopic levels. Reprinted with permis- sion from Oatis CA. Kinesiology: The Mechanics and Pathomechanics of Human Movement. Philadelphia, Pa: Lippincott Williams & Wilkins; 2004:46. which contains only actin (thin) filaments. The darker area is the A-band, which contains alternating actin and myosin (thick) filaments. The Z-line consists of a connective tissue network that bisects the I-band, anchors the thin filaments, and provides structural integrity to the sarcomere. The H- Figure 3-1. Successive connective tissue sheaths with- zone, located in the middle of the A-band, is the region of in muscle. Reprinted with permission from Oatis thick filaments not overlapped by thin filaments. The M- CA. Kinesiology: The Mechanics and Pathomechanics band bisects the H-zone and represents the middle of the of Human Movement. Philadelphia, Pa: Lippincott sarcomere. The M-band consists of protein structures that Williams & Wilkins; 2004:47. support the arrangement of the myosin filaments. During muscle contraction, the sarcomere I-band and H-zone decrease in length while the length of the A-band remains constant.2,3 THE MUSCLE SPINDLE The muscle spindle is a long, thin structure located adjacent and parallel to muscle fibers and is composed of multiple components that have both afferent and efferent innervation (Figures 3-4a and 3-4b). The muscle spindle functions as a stretch receptor and responds to static and dynamic length changes of skeletal muscle.4-6 This complex receptor is found in all muscles, primarily in extremity, inter- costal, and cervical muscles.