Homeopathic Medicine & Advertising Workshop Transcript
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Homeopathy and Psychological Therapies
Entry Homeopathy and Psychological Therapies Davide Donelli * and Michele Antonelli AUSL-IRCCS Reggio Emilia, 42122 Reggio Emilia, Italy; [email protected] * Correspondence: [email protected] Definition: Homeopathy is a popular, although highly debated, medicinal practice based on the administration of remedies in which active substances are so diluted that no detectable trace of them remains in the final product. This hypothesis paper aims to outline a possible reinterpreta- tion of homeopathy in the light of psychological therapies in order to improve its clinical safety and sustainability. Keywords: homeopathy; psychology; reinterpretation; hypothesis 1. Introduction Homeopathy is a popular, although highly debated, medicinal practice. In Italy, for ex- ample, it is estimated that, even if with a slightly declining trend, around 4.1% of the entire population (almost 2.5 million people) occasionally or regularly seeks homeopathic care, and these data, collected in 2013, suggest that homeopathy is the most used Complemen- tary and Alternative Medicine (CAM) by Italians [1]. Epidemiological studies aimed to assess the worldwide prevalence of homeopathy use have reported similar data for other high-income countries [2]. Homeopathy was first invented by the German doctor Samuel Hahnemann (1755–1843), and it is based on the administration of remedies in which active substances are so diluted that no detectable trace of them remains in the final product [3]. In his empirical studies, Citation: Donelli, D.; Antonelli, M. Hahnemann reported that the self-administration of a common antimalarial medicinal Homeopathy and Psychological Ther- plant (Cinchona) resulted in the occurrence of the same symptoms of malaria, but to a Encyclopedia 2021 1 apies. -

Homeopathy Time Tested Remedies for the Whole Family Safe and Effective
magazine presents homeopathy Time TesTed remedies for the whole family safe and effective $4.95 magazine presents HomeopatHy by kim erickson Get Healthy with HomeopatHy! Based on the premise that “like cures like,” homeopathy is a terrific way to help the whole family stay healthy. But if you’re not familiar with this safe and gentle mode of healing, you’re certainly not alone. For those of us in the U.S., it may be one of the least familiar forms of natural medicine, yet it can be one of the most effective. According to the World Health Organization more than 500 million people worldwide use homeopathy. It’s especially popular in Europe, India, and South Kim Erickson America. Why? Because it works! Managing Editor Best of all, many homeopathic remedies are ideal for handling many of life’s little everyday health con- cerns, whether it’s a bout with hay fever, a sprained ankle, or the common cold. This booklet will give you an in-depth understanding of how homeopathy sup- ports good health and why it may be the right choice for you and your family. AMAZING wellness™ Check out the latest issue of Amazing Wellness magazine at your local Vitamin Shoppe or at www.amazingwellnessmag.com www.vitaminshoppe.com Copyright © 2011 by Kim Erickson and Active Interest Media, Inc. All rights reserved. No part of this booklet may be reproduced, stored in an electronic retrieval system, or transcribed in any form or by any means, electronic or mechanical, including photocopying and recording, without the prior written permission of the publisher, except for the inclusion of quotations in a review. -

Introduction to Homeopathy for the Primary Care Nurse Practitioner
Introduction to Homeopathy for the Primary Care Nurse Practitioner Linda Goldman DNP FNP WHNP-BC CANP Annual Conference March, 2014 Powerpoint Templates Page 1 Dr. Goldman has the following information to disclose: : • Financial Disclosure – Speakers Bureau: Boiron USA - A company that manufacturers Homeopathic Medicines • FDA approved indications for use – All medications discussed during today’s presentation will comply with the FDA labeling requirements for approved indications according to the Homeopathic Pharmacopeia of the United States (HPUS). Powerpoint Templates Page 2 Objectives By the end of this discussion the participant will be able to: 1. Differentiate homeopathy from herbal and other complementary and alternative modalities 2. Identify appropriate indications, side effects, and dosages of commonly used homeopathic agents 3. Analyze the regulations and production practices of companies producing homeopathic agentsPowerpoint Templates Page 3 Complementary & Alternative Medicine (CAM) Definition: “CAM is a group of diverse medical and health care systems, practices, and products that are not generally considered to be part of conventional medicine” http://nccam.nih.gov/health/homeopathy/#q606070 9 Powerpoint Templates Page 4 Complementary & Alternative Medicine (CAM) • National Center for Complementary & Alternative Medicine (NCCAM) – Division of the NIH – Funding for research – http://nccam.nih.gov • Centers for Disease Control CDC – www.cdc.gov • Institute of Medicine (IOM) published review – www.iom.edu Powerpoint Templates -

Evidence Check 2: Homeopathy
House of Commons Science and Technology Committee Evidence Check 2: Homeopathy Fourth Report of Session 2009–10 HC 45 House of Commons Science and Technology Committee Evidence Check 2: Homeopathy Fourth Report of Session 2009–10 Report, together with formal minutes, oral and written evidence Ordered by the House of Commons to be printed 8 February 2010 HC 45 Published on 22 February 2010 by authority of the House of Commons London: The Stationery Office Limited £0.00 The Science and Technology Committee The Science and Technology Committee is appointed by the House of Commons to examine the expenditure, administration and policy of the Government Office for Science. Under arrangements agreed by the House on 25 June 2009 the Science and Technology Committee was established on 1 October 2009 with the same membership and Chairman as the former Innovation, Universities, Science and Skills Committee and its proceedings were deemed to have been in respect of the Science and Technology Committee. Current membership Mr Phil Willis (Liberal Democrat, Harrogate and Knaresborough)(Chairman) Dr Roberta Blackman-Woods (Labour, City of Durham) Mr Tim Boswell (Conservative, Daventry) Mr Ian Cawsey (Labour, Brigg & Goole) Mrs Nadine Dorries (Conservative, Mid Bedfordshire) Dr Evan Harris (Liberal Democrat, Oxford West & Abingdon) Dr Brian Iddon (Labour, Bolton South East) Mr Gordon Marsden (Labour, Blackpool South) Dr Doug Naysmith (Labour, Bristol North West) Dr Bob Spink (Independent, Castle Point) Ian Stewart (Labour, Eccles) Graham Stringer (Labour, Manchester, Blackley) Dr Desmond Turner (Labour, Brighton Kemptown) Mr Rob Wilson (Conservative, Reading East) Powers The Committee is one of the departmental Select Committees, the powers of which are set out in House of Commons Standing Orders, principally in SO No.152. -

Issn 1475-49 16
Volume 94 • Number 3 July 2005 ISS N 1475-49 16 • RCT of homeopathy for depression • lnfection, vaccination and atopy • Systematic review of homeopathy in depression • The colour of improvement • Plumbum and proving reproducibility • Vaccine reactions and vaccinosis • Homeopathy and dry mOuth • A systematic approach to the Organon Editor Peter Fisher FRCP FFHom London, UK Editorial Board Bob Leckridge Robert T Mathie Tom Whitmarsh MBChBFFHom BSc (Hans), PhD MA MBBS FRCP FFHom Editorial Advisory Board Cees Baas Edzard Ernst Luisa Queralt Artsenpraktijk voor Horneapathie University of Exeter, UK Acadernia Medico Horneopatica Eindhoven, The Netherlands de Barcelona, Spain Edoardo Felisi Madeleine Bastide CISDO, Milan, ltaly University of Montpellier, France DPRastogi Gentraf Council for Research Iris R Bell Trevor Gibbs in Hornoeopathy, lndia The University of Arizona, USA University of Cape Town, South Africa David Reilly Philippe Belon Robert Gilchrist Glasgow Hornoeopathic Hospital, UK Institut Boiron, France University of North London, UK David Riley Zvi Bentwich German Guajardo-Bernal Rosetta Genornics, Israel University of New Mexico Medical School, University of Baja California, Mexico USA Brian Berman University of Maryland, USA Marianne Heger Horn/nt Research Centre, Gerrnany Jurgen Schulte Martien Brands University of Technology, Sydney, Australia University of Uverpool, UK Jennifer Jacobs University of Washington, USA Michael Carlston TrevorThompson University of California San Francisco, USA Wayne Jonas University of Bristol, -
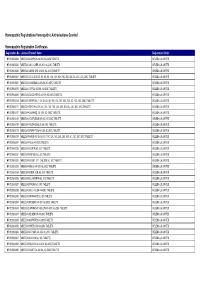
HR & NR Listing with Product Name (FINAL) 18-05-2021
Homeopathic Registrations/Homeopathic Authorisations Granted Homeopathic Registration Certificates Registration No. Licensed Product Name Registration Holder HR 00298/0006 WELEDA ACID PHOS 4X-30X, 6C-200C TABLETS WELEDA UK LIMITED HR 00298/0020 WELEDA CALC. CARB. 4X-30X, 6C-200C TABLETS WELEDA UK LIMITED HR 00298/0033 WELEDA CARBO VEG 4X-30X, 6C-200C TABLETS WELEDA UK LIMITED HR 00298/0047 WELEDA COCCULUS 4X, 5X, 6X, 8X, 10X, 12X, 15X, 20X, 25X, 30X, 6C, 12C, 30C, 200C TABLETS WELEDA UK LIMITED HR 00298/0061 WELEDA CHAMOMILLA 4X-30X, 6C-200C TABLETS WELEDA UK LIMITED HR 00298/0075 WELEDA COFFEA 4X-30X, 6C-200C TABLETS WELEDA UK LIMITED HR 00298/0089 WELEDA COLOCYNTHIS 4X-30X, 6C-200C TABLETS WELEDA UK LIMITED HR 00298/0103 WELEDA HEPAR SULF. 4X, 5X, 6X, 8X, 10X, 15X, 20X, 25X, 30X, 6C, 12C, 30C, 200C TABLETS WELEDA UK LIMITED HR 00298/0117 WELEDA IGNATIA 4X, 5X, 8X, 10X, 12X, 15X, 20X, 25X, 30X, 6C, 12C, 30C, 200C TABLETS WELEDA UK LIMITED HR 00298/0131 WELEDA KALI PHOS. 4X- 30X, 6C- 200C TABLETS WELEDA UK LIMITED HR 00298/0145 WELEDA LYCOPODIUM 4X-30X, 6C-200C TABLETS WELEDA UK LIMITED HR 00298/0159 WELEDA PHOSPHOROUS 4X-200C TABLETS WELEDA UK LIMITED HR 00298/0173 WELEDA SYMPHYTUM 4X-30X, 6C-200C TABLETS WELEDA UK LIMITED HR 00298/0187 WELEDA TAMUS 4X, 5X, 6X, 8X, 10X, 12X, 15X, 20X, 25X, 30X, 6C, 12C, 30C, 200C TABLETS WELEDA UK LIMITED HR 00298/0201 WELEDA THUJA 4X-200C TABLETS WELEDA UK LIMITED HR 00298/0228 WELEDA ACONITE 6C, 30C TABLETS WELEDA UK LIMITED HR 00298/0231 WELEDA APIS MEL 6C, 30C TABLETS WELEDA UK LIMITED HR 00298/0232 WELEDA ARGENT. -

Homeopathic Prevention and Treatment of Epidemics & Pandemics
Homeopathic Prevention & Treatment of Epidemics & Pandemics Sharum Sharif, ND American Association of Naturopathic Physicians August of 2019, Portland, Oregon Cholera, a Horrific Present-Day Epidemic • Cholera killed 1.1 million people in Yemen in just 2017 alone. • How BIG is 1.1 million? That’s approximately the population of the following cities in WA: Seattle(725K), Bellevue(144K), Renton(101K), kirkland(88K), & Redmond(64K) • 1.1m in one year = 3090 people PER DAY! (Note: Malaria also kills 3000 children/day.) • The national average public school student size is approximately 525 students (2018-19). So, 1.1 million in one year = 3090/day = number of Kids in 6 U.S. publiC schools/day. Why should we discuss epidemics and why should we prepare ourselves for them ? •Epidemics are on the rise, and they will be one of the main challenges we will face as a species increasingly over the next few years and in the future. •The biggest killers in history have been epidemic diseases. And, that is why we should prepare ourselves for them. What are epidemic & pandemic diseases? • EndemiC: Regularly found among particular people or in a certain area, and it goes on through generations. Example: • Cholera has been endemic in India for 2000 years. • Plague is endemic in the Western U.S., South America, Asia and Africa. • EpidemiC: Much more acute than endemic. A widespread occurrence of an infectious disease in a community at a particular time. • PandemiC: Prevalent over a whole country or the world. What sort of epidemics are we referring to here? True epidemics are acute infectious diseases. -

View Fall 2020 Issue Here
Your Guide to Health through Homeopathy BACK TO SCHOOL 2020 What if my kid L VES being at home? TEACHING KIDS HOMEOPATHY IT’S CONTINUES HERE! EVEN MORE GREAT LESSON 2020 BUYER’S PLANS AND WORKSHEETS! GUIDE Rheumatoid Arthritis Pain—Relieved! Remembering A FARMER’S HEALING JOURNEY Julian “Winnie” Winston 15th Anniversary Tribute Alice Beats Shortness of Breath, Tosses Away Walker A COMMON REMEDY RESTORES HEALTH PARVO—A VIRAL PUPPY PANDEMIC HOW HOMEOPATHY CAN HELP Fall 2020 • $11.95 HomeopathyCenter.org Call today and speak to one of our friendly customer service reps. Join us and get your FREE illustrated catalogs and topic-specific pamphlets, either one-time or regularly by joining our mailing list. Choose us for easy one-stop shopping of major homeopathic brands like Hyland’s, Boiron, Similisan, Homeopet, Bach, Weleda and more! Contact us to obtain live excellent service every time, with our experienced customer service representatives, averaging over 11 years of service! Browse us at 1800Homeopathy.com to flip through our electronic catalogs, find articles, read reviews, and browse through hundreds of healing products for babies, kids, tweens, teens and adults. 1-800-466-3672 www.1800homeopathy.com Product claims are based on traditional homeopathic practice, not accepted medical evidence. Not FDA evaluated. Stay Connected! Fall 2020 Volume 40, Number 3 ISSN: 0886-1676 www.HomeopathyCenter.org Your Guide to Health through Homeopathy Featured Articles Back to School 2020 16 What if my kid LOVES being at home? By Amy Rothenberg, ND, DHANP -

Fda's Homeopathic Risk-Based Enforcement
JAMES MACRO DRAFT (DO NOT DELETE) 12/6/20 9:47 AM FDA’S HOMEOPATHIC RISK-BASED ENFORCEMENT: COMPROMISED CONSUMER PROTECTION OR STEPPED-UP SCRUTINY? Carrie Scrufari James† TABLE OF CONTENTS ABSTRACT .......................................................................................... 1116 INTRODUCTION ................................................................................... 1117 I. HISTORICAL OVERVIEW OF DRUG REGULATION IN THE UNITED STATES ..................................................................................... 1120 A. Undiluted Fraud ............................................................... 1121 B. State Attempts While Quacks Abound .............................. 1123 C. The 1906 Pure Foods and Drugs Act ............................... 1124 D. The Federal Food Drug and Cosmetics Act of 1938 ....... 1133 1. The 1951 Durham-Humphrey Amendment ................. 1137 2. The 1962 Kefauver-Harris Amendment ...................... 1139 3. 1972 GRASE Review of OTC Drugs .......................... 1141 4. OTC Monograph Review Process .............................. 1142 A. Premarket Approval Process of Monographed OTCs .............................................................................. 1143 B. Post-market Review of Monographed OTCs ........ 1144 C. OTC Exclusions: Homeopathic Drugs ................. 1144 5. NDA Approval Process for Prescription Drugs ......... 1145 II. REGULATING HOMEOPATHIC PRODUCTS .................................. 1146 A. Theory, Practice, and Principles .................................... -

What About Homeopathy?
What about Homeopathy? A comparative investigation into the causes of current popularity of homeopathy in The USA, The UK, India and The Netherlands Dulcia non meruit qui non gustavit amara ____________________________ MA Comparative History Thesis | J.T.H.J. Dekkers 1 What about Homeopathy? A comparative investigation into the causes of current popularity of homeopathy in The USA, The UK, India and The Netherlands This Master of Arts Comparative History Thesis Is Written Under the Authority of the University of Utrecht First Supervisor: dr. Fia Dieteren Second Supervisor: dr. Joost Vijselaar Third Supervisor: prof. dr. Martin Dinges Overall Supervisor and Master Program Director: prof. dr. Maarten Prak Author: Joris Theodorus Hubertus Johannes Dekkers First Edition: July 2009 (Nuenen) Copyshop Rooijmans Nuenen © 2009 Nothing in this publication is intended to reduce, limit, or restrict any rights arising from fair use, first sale or other limitations on the exclusive rights of the copyright owner under copyright law or other applicable laws. Nothing in this license is intended to reduce, limit, or restrict any rights owned by third-parties. No content may be copied, made public, spread or used without the express written permission of the author. 2 Contents Preface and Acknowledgements………………………………………………………………………………………… 4 Introduction……………………………………………………………………………………………………………………….. 6 Chapter 1 | The USA……………………………………………………………………………………………………….…… 20 Chapter 2 | The UK..……………………………………………………………………………………………………….…… 28 Chapter 3 | India…...……………………………………………………………………………………………….…………… -

An Alternative to Vaccines? Nosodes and Their Effect on Vaccine Debates in English Canada
AN ALTERNATIVE TO VACCINES? NOSODES AND THEIR EFFECT ON VACCINE DEBATES IN ENGLISH CANADA A thesis submitted to the College of Graduate and Postdoctoral Studies In partial fulfilment of the requirements for MASTER OF ARTS in the Department of History UNIVERSITY OF SASKATCHEWAN Saskatoon by Derek Hilliard Cameron Copyright © Derek Cameron, February 2020 All Rights Reserved Permission to Use In presenting this thesis/dissertation in partial fulfilment of the requirements for a Postgraduate degree from the University of Saskatchewan, I agree that the Libraries of this University may make it freely available for inspection. I further agree that permission for copying of this thesis/dissertation in any manner, in whole or in part, for scholarly purposes may be granted by the professor or professors who supervised my thesis/dissertation work or, in their absence, by the Head of the Department or the Dean of the College in which my thesis work was done. It is understood that any copying or publication or use of this thesis/dissertation or parts thereof for financial gain shall not be allowed without my written permission. It is also understood that due recognition shall be given to me and to the University of Saskatchewan in any scholarly use which may be made of any material in my thesis/dissertation. Requests for permission to copy or to make other uses of materials in this thesis/dissertation in whole or part should be addressed to: Head of the Department of History HUMFA Administrative Support Services Room 522, Arts Building University of Saskatchewan 9 Campus Drive Saskatoon, Saskatchewan, Canada S7N 5A5 OR Dean College of Graduate and Postdoctoral Studies University of Saskatchewan 116 Thorvaldson Building, 110 Science Place Saskatoon, Saskatchewan S7N 5C9 Canada i Abstract In the 1980s, vaccine hesitance created a market for vaccine alternatives in Canada. -

Staff Report on the Homeopathic Medicine & Advertising Workshop
Staff Report on the Homeopathic Medicine & Advertising Workshop Federal Trade Commission November 2016 Homeopathic Medicine & Advertising Workshop Report Federal Trade Commission Edith Ramirez, Chairwoman Maureen K. Ohlhausen, Commissioner Terrell McSweeny, Commissioner Table of Contents I. Introduction …………………………………………………………………………...…1 II. Summary of the Homeopathic Medicine & Advertising Workshop……………….…2 A. Panel 1: Homeopathic Industry & Advertising………………………………....…2 B. Panel 2: Evaluating the Scientific Support for Homeopathic Advertising Claims..5 C. Panel 3: Legal and Regulatory Issues Presented by Homeopathic Advertising…..8 III. FTC’s Homeopathic Consumer Research………………………………...…………..11 A. Focus Group Results……………………………………………………...………….11 B. Copy Test Results…...…………………………………………………...…………..12 IV. Public Comments………………………………………………………………….……14 A. Comments from Individual Users or Practitioners of Homeopathy………………....14 B. Comments from Individual Skeptics of Homeopathy……………………….……....14 C. Comments from Organizations Skeptical of Homeopathy………………..………....14 D. Comments from a Homeopathic Manufacturer…………………………..…….…....15 E. Comments from Organizations Representing Homeopathic Companies or Practitioners…………………………………………..………………………...…....16 1. The Homeopathic Nurses Association……………………………………...…....16 2. The United States Homeopathic Regulatory Commission……………………....16 3. The American Association of Homeopathic Pharmacists……………….……....17 a. The first AAHP study………………………………………………………..19 b. The second AAHP study………………………………………………….....20