Marine Fisheries Information Service Technical & Extension Series
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Studies on Coastal Geomorphology Along Visakhapatnam to Bhimunipatnam, East Coast of India M
J. Ind. Geophys. Union ( October 2012 ) Vol.16, No.4, pp. 179-187 Studies on Coastal Geomorphology along Visakhapatnam to Bhimunipatnam, East Coast of India M. Jagannadha Rao, Greeshma Gireesh A.G., P. Avatharam1, N.C. Anil2 and T. Karuna Karudu Delta Studies Institute, 1Dept of Geology, 2Dept. of Geo-Engineering, Andhra University, Visakhapatnam. ABSTRACT An attempt has been made to study and record the diverse coastal geomorphic features along Visakhapatnam – Bhimunipatnam coast. The study area is marked by geomorphic features resulted from various coastal and land ward processes. The geomorphic units under different heads namely, features formed by present day wave action, features formed due to sea level oscillations and features formed by rock and sea water interaction have been presented. The influence of sea level oscillations and land and sea interaction forms a diverse coastal geomorphology along the coast. The details of features such as beach, dune system, inter tidal deposits, red sediments, wave cut platform, marine terrace, sea stack, sea cave, serpent hood structures, promontories, wind gap and natural bridge have been recorded and the formation as well as significance of their occurrence are presented. The coastal geomorphology of the study area clearly established not only the sea level oscillations but also variations in climatic conditions in this part of the coast. INTRODUCTION PHYSIOGRAPHY Coastal geomorphology by definition is the study of The study area is characterized by Eastern Ghat the morphological development and evolution of the mobile belt. This area covered with denudational coast as it acts under the influence of winds, waves, hills of range between 30 to 594m above mean currents and sea level changes. -

List of Village Panchayats in Tamil Nadu District Code District Name
List of Village Panchayats in Tamil Nadu District Code District Name Block Code Block Name Village Code Village Panchayat Name 1 Kanchipuram 1 Kanchipuram 1 Angambakkam 2 Ariaperumbakkam 3 Arpakkam 4 Asoor 5 Avalur 6 Ayyengarkulam 7 Damal 8 Elayanarvelur 9 Kalakattoor 10 Kalur 11 Kambarajapuram 12 Karuppadithattadai 13 Kavanthandalam 14 Keelambi 15 Kilar 16 Keelkadirpur 17 Keelperamanallur 18 Kolivakkam 19 Konerikuppam 20 Kuram 21 Magaral 22 Melkadirpur 23 Melottivakkam 24 Musaravakkam 25 Muthavedu 26 Muttavakkam 27 Narapakkam 28 Nathapettai 29 Olakkolapattu 30 Orikkai 31 Perumbakkam 32 Punjarasanthangal 33 Putheri 34 Sirukaveripakkam 35 Sirunaiperugal 36 Thammanur 37 Thenambakkam 38 Thimmasamudram 39 Thilruparuthikundram 40 Thirupukuzhi List of Village Panchayats in Tamil Nadu District Code District Name Block Code Block Name Village Code Village Panchayat Name 41 Valathottam 42 Vippedu 43 Vishar 2 Walajabad 1 Agaram 2 Alapakkam 3 Ariyambakkam 4 Athivakkam 5 Attuputhur 6 Aymicheri 7 Ayyampettai 8 Devariyambakkam 9 Ekanampettai 10 Enadur 11 Govindavadi 12 Illuppapattu 13 Injambakkam 14 Kaliyanoor 15 Karai 16 Karur 17 Kattavakkam 18 Keelottivakkam 19 Kithiripettai 20 Kottavakkam 21 Kunnavakkam 22 Kuthirambakkam 23 Marutham 24 Muthyalpettai 25 Nathanallur 26 Nayakkenpettai 27 Nayakkenkuppam 28 Olaiyur 29 Paduneli 30 Palaiyaseevaram 31 Paranthur 32 Podavur 33 Poosivakkam 34 Pullalur 35 Puliyambakkam 36 Purisai List of Village Panchayats in Tamil Nadu District Code District Name Block Code Block Name Village Code Village Panchayat Name 37 -

Academic Calendar 19-20
Academic Calendar 2019-20 TBAK COLLEGE FOR WOMEN THASSIM BEEVI ABDUL KADER COLLEGE FOR WOMEN Kilakarai - 623 517, Ramanathapuram District Sponsored by Seethakathi Trust, Chennai - 600 006 [A Minority Autonomous Institution & Re-accredited by NAAC with B++ Grade ISO 9001:2015 Certified Institution] Affiliated to Alagappa University, Karaikudi 2 0 1 9 - 2 0 1 TBAK COLLEGE FOR WOMEN Academic Calendar 2019-20 In the name of the Almighty, The Most Gracious, The Most Merciful! All praise be to the Almighty only! Towards the end of the meeting recite this together with the audience Glory be to the Almighty and praise be to Him! Glory be to YOU and all praise be to You! I bear witness that there is no true GOD except YOU alone. I ask your pardon and turn to YOU in repentance. [Dua from the Hadith of Prophet Muhammad (PBUH) Narrated by Abu Hurairah (Rali) Source: Abu Dawud: 4859] 2 Academic Calendar 2019-20 TBAK COLLEGE FOR WOMEN In the Name of Allah, the Most Gracious, the Most Merciful Proclaim (Or Read :) In the name of thy Lord and Cherisher, Who Created man, out of a mere clot of congealed blood. Proclaim! And thy Lord is Most Bountiful He who taught the use of the pen Taught man that which he knew not Nay, but man doth transgress all bounds In that he looketh upon himself as self-sufficient. Verily, to thy Lord is the return of all. Alquran Sura 96: (verses 1 to 8) 3 TBAK COLLEGE FOR WOMEN Academic Calendar 2019-20 Founded in 1988 G O No 1448 dated 12 September 1988 THASSIM BEEVI ABDUL KADER COLLEGE FOR WOMEN (Sponsored by Seethakathi Trust, Chennai) (Affiliated to Alagappa University, Karaikudi) No. -
S.No. Shop Address 1 Anna Nagar Shanthi Colony
S.No. Shop Address Anna Nagar Shanthi Colony Aa-144, 2nd Floor, 3rd Avenue, (Next To Waves) Anna Nagar, Ch-600040. 1 Anna Nagar West No 670,Sarovar Building, School Road, Anna Nagar West, Chennai - 600101. 2 Mogappair East 4/491, Pari Salai, Mogappair East, (Near Tnsc Bank) Ch-600037 3 Mogappair West 1 Plot No.4, 1st Floor, Phase I, Nolambur,(Near Reliance Fresh) Mogappair 4 West, Ch-600037. Annanagar West Extn Plot No: R48, Door No - 157, Tvs Avenue Main Road,Anna Nagar West 5 Extension,Chennai - 600 101. Opp To Indian Overseas Bank. Red Hills 1/172a, Gnt Road, 2nd Floor, Redhills-Chennai:52. Above Lic, Next To Iyappan 6 Temple K.K.Nagar 2 No.455, R.K.Shanuganathan Road, K K Nagar, Land Mark:Near By K M 7 Hospital, Chennai - 600 078 Tiruthani No. 9, Chittoor Road, Thirutani - 631 209 8 Anna Nagar (Lounge) C Block, No. 70, Tvk Colony, Annanagar East, Chennai - 102. 9 K.K.Nagar 1 Plot No 1068, 1st Floor, Munuswami Salai, (Opp To Nilgiri Super Market) 10 K.K.Nagar West, Ch-600078. Alapakkam No. 21, 1st Floor, Srinivasa Nagar,Alapakkam Main 11 Road,Maduravoyal,Chennai 600095 Mogappair West 2 No-113, Vellalar Street, Mogappair West, Chennai -600 037. 12 Poonamalle # 35, Trunk Road, Opp To Grt Poonamalle Chennai-600056. 13 Karayanchavadi N0. 70, Trunk Road, Karayanchavadi, Poonamallee, Chennai - 56 14 Annanagar 6th Avenue 6th Avenue,Anna Nagar,Chennai 15 Chetpet Opp To Palimarhotel,73,Casamajorroad,Egmore,Ch-600008 16 Egmore Lounge 74/26,Fagunmansion,Groundfloor,Nearethirajcollege,Egmore,Chennai-600008 17 Nungambakkam W A-6, Gems Court, New.25 (Old No14), Khader Nawaz Khan Road, (Opp Wills 18 Life Style) Nungambakkam, Ch-600034. -

ANSWERED ON:11.05.2005 AUTOMATIC and MODERN TELEPHONE EXCHANGES in TAMIL NADU Kharventhan Shri Salarapatty Kuppusamy
GOVERNMENT OF INDIA COMMUNICATIONS AND INFORMATION TECHNOLOGY LOK SABHA UNSTARRED QUESTION NO:6879 ANSWERED ON:11.05.2005 AUTOMATIC AND MODERN TELEPHONE EXCHANGES IN TAMIL NADU Kharventhan Shri Salarapatty Kuppusamy Will the Minister of COMMUNICATIONS AND INFORMATION TECHNOLOGY be pleased to state: (a) the details of automatic and modern telephone exchanges set up in Tamil Nadu during the last three years, location- wise; (b) the details of such exchanges proposed to be set up in Tamil Nadu during the current year; (c) the details of the telephone exchanges whose capacities were expanded in the current financial year; and (d) the details of telephone exchanges where waiting list for telephone connection still exists? Answer THE MINISTER OF STATE IN THE MINISTRY OF COMMUNICATIONS ANDINFORMATION TECHNOLOGY (DR. SHAKEEL AHMAD) (a) The details of automatic and modern telephone exchanges set up in Tamilnadu during the last three years are given in the Annexures- I(a), I(b) & I(c). (b) The details of such exchanges proposed to be set up in Tamilnadu during the current year are given in Annexure-II. (c) The details of the telephone exchanges whose capacities were expanded in the current financial year are given at Annexure-III. (d) The details of telephone exchanges where waiting list for telephone connection still exists are given in Annexure- IV. ANNEXURE-I(a) DETAILS OF TELEPHONE EXCHANGES SET UP DURING 2002-03 IN TAMILNADU Sl Name of Exchange Capacity Type/Technology District No.(Location) 1 Avinashi-II 4000 CDOTMBMXL Coimbatore 2 K.P.Pudur -
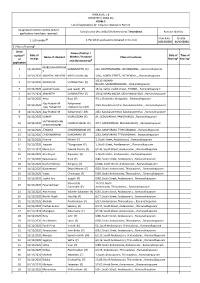
ANNEXURE 5.8 (CHAPTER V, PARA 25) FORM 9 List of Applica Ons For
ANNEXURE 5.8 (CHAPTER V, PARA 25) FORM 9 List of Applicaons for inclusion received in Form 6 Designated locaon identy (where Constuency (Assembly/£Parliamentary): Tiruvadanai Revision identy applicaons have been received) From date To date @ 2. Period of applicaons (covered in this list) 1. List number 01/12/2020 01/12/2020 3. Place of hearing* Serial Name of Father / $ Date of Date of Time of number Name of claimant Mother / Husband Place of residence of receipt hearing* hearing* and (Relaonship)# applicaon MURUGANANTHAM 1 01/12/2020 SONAIMUTHU (F) 222, KAATHAANVAYAL, IRUVANIVAYAL, , Ramanathapuram S 2 01/12/2020 ANANTHI ANANTHI MEGALAIVAN (H) 1/36,, NORTH STREET,, PUTHENDAL,, , Ramanathapuram 3/310, INDARA 3 01/12/2020 GOWSALYA VAIYAMUTHU (F) NAGAR, SADAIYANVALASAI, , Ramanathapuram 4 01/12/2020 ayeshath fazila asiq rasool (F) 13/1a, bathu shahib street , THONDI, , Ramanathapuram 5 01/12/2020 ARAVINTH VAIYAMUTHU (F) 3/310, INDRA NAGAR, SADAIYANVALASAI, , Ramanathapuram 6 01/12/2020 Anbu Rasu (F) 4/11, Sholandur, Mangulam, , Ramanathapuram Ajay Prakash M Kaliyammal 7 01/12/2020 2/63, Kanakakanenthal, Kanakakanenthal, , Ramanathapuram Ajay Prakash M J Kaliyammal J (M) 8 01/12/2020 Ajay Prakash M Kaliyammal J (M) 2)63, Kanakakanenthal, Kanakakanenthal, , Ramanathapuram 9 01/12/2020 KUMAR MURUGESAN (F) 84, NEDUMARAM, PANICHAKUDI, , Ramanathapuram VAITHIYANATHAN 10 01/12/2020 CHINNATHAMBI (F) 3/75, SAMPOORANI, MANGALAKUDI, , Ramanathapuram CHINNATHAMBI 11 01/12/2020 JEYAKODI CHIDAMBARAM (H) 3/82, SAMPURANI, THIRUVADANAI, , Ramanathapuram -

Tamil Nadu H2
Annexure – H 2 Notice for appointment of Regular / Rural Retail Outlet Dealerships IOCL proposes to appoint Retail Outlet dealers in the State of Tamil Nadu as per following details: Name of location Estimated Minimum Dimension (in Finance to be Fixed Fee / monthly Type of Mode of Security Sl. No Revenue District Type of RO Category M.)/Area of the site (in Sq. arranged by the Minimum Sales Site* Selection Deposit M.). * applicant Bid amount Potential # 1 2 3 4 5 6 7 8 9a 9b 10 11 12 (Regular/Rural) (SC/SC CC (CC/DC/CFS) Frontage Depth Area Estimated Estimated (Draw of Rs. in Lakhs Rs. in 1/SC PH/ST/ST working fund Lots/Bidding) Lakhs CC 1/ST capital required PH/OBC/OBC requireme for CC 1/OBC nt for developme PH/OPEN/OPE operation nt of N CC 1/OPEN of RO Rs. in infrastruct CC 2/OPEN Lakhs ure at RO PH) Rs. in Lakhs 1 Alwarpet Chennai Regular 150 SC CFS 20 20 400 0 0 Draw of Lots 0 3 2 Andavar Nagar to Choolaimedu, Periyar Pathai Chennai Regular 150 SC CFS 20 20 400 0 0 Draw of Lots 0 3 3 Anna Nagar Chennai Regular 200 Open CC 20 20 400 25 10 Bidding 30 5 4 Anna Nagar 2nd Avenue Main Road Chennai Regular 200 SC CFS 20 20 400 0 0 Draw of Lots 0 3 5 Anna Salai, Teynampet Chennai Regular 250 SC CFS 20 20 400 0 0 Draw of Lots 0 3 6 Arunachalapuram to Besant nagar, Besant ave Road Chennai Regular 150 SC CFS 20 20 400 0 0 Draw of Lots 0 3 7 Ashok Nagar to Kodambakam power house Chennai Regular 150 SC CFS 20 20 400 0 0 Draw of Lots 0 3 8 Ashok Pillar to Arumbakkam Metro Chennai Regular 200 Open DC 13 14 182 25 60 Draw of Lots 15 5 9 Ayanavaram -

OVERVIEW of VUDA, VISAKHAPATNAM the Town Planning Trust (TPT) Was Constituted in 1962 and the Visakhapatnam Urban Development Au
OVERVIEW OF VISAKHAPATNAM UDA'S ACTIVITIES OVERVIEW OF VUDA, VISAKHAPATNAM The Town Planning Trust (TPT) was constituted in 1962 and the Visakhapatnam Urban Development Authority comes into existence in 1978 under AP Urban Areas (Dev) Act, 1975 for the areas covering Visakhapatnam, Gajuwaka, Bheemunipatnam, Anakapalle and Vizianagaram Municipalities and 287 villages with an extent of 1721 Sq.Kms. The VMR Region has been witnessing tremendous growth and accordingly, the Government vide GO.Ms.No. 525 of MA & UD Dept, dt. 30-7-2008 have extended the jurisdiction of VUDA to 5573 Sq.Kms covering four Districts of Srikakulam, Vizianagaram, Visakhapatnam & East Godavari and Municipalities of Amudalavalasa, Srikakulam of Srikakulam District & Tuni of East Godavari District The Government have constituted the VUDA Bpard with the following members (viic CO.Ms.No. 373, dt 27-8-2011 of MA & UD Department) : 1 The Principal Secretary, MA&UD Department GoAP Chairman 2 The Additional Secretary to Govt, Finance Department Member (W&P) 3 The Member Secretary, Andhra Pradesh Pollution Control Ex-officio Member Board 4 The Managing Director, Andhra Pradesh Industrial Ex-officio Member Infrastructure Corporation 5 The Commissioner & Managing Director, A.P. Eastern Ex-officio Member Power Distribution Corporation Ltd., C< The District Collector, Visakhapatnam Ex-officio Member 7 The District Collector, Srikakulam Ex-officio Member 8 The District Collector, Vizianagaram Ex-officio Member 9 The District Collector, East Godavari Ex-officio Member 10 The Commissioner, Greater Visakhapatnam Municipal Member Corporation 11 The Director of Town & Country Planning Member 12 The Vice Chairman, Visakhapatnam Urban Development Member-Convener Authority At present there are 215 employees working in respect of all cadres, out of the sar-tioned strength of 316. -
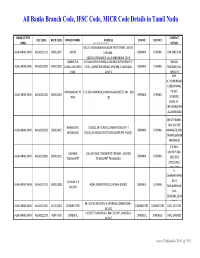
Banks Branch Code, IFSC Code, MICR Code Details in Tamil Nadu
All Banks Branch Code, IFSC Code, MICR Code Details in Tamil Nadu NAME OF THE CONTACT IFSC CODE MICR CODE BRANCH NAME ADDRESS CENTRE DISTRICT BANK www.Padasalai.Net DETAILS NO.19, PADMANABHA NAGAR FIRST STREET, ADYAR, ALLAHABAD BANK ALLA0211103 600010007 ADYAR CHENNAI - CHENNAI CHENNAI 044 24917036 600020,[email protected] AMBATTUR VIJAYALAKSHMIPURAM, 4A MURUGAPPA READY ST. BALRAJ, ALLAHABAD BANK ALLA0211909 600010012 VIJAYALAKSHMIPU EXTN., AMBATTUR VENKATAPURAM, TAMILNADU CHENNAI CHENNAI SHANKAR,044- RAM 600053 28546272 SHRI. N.CHANDRAMO ULEESWARAN, ANNANAGAR,CHE E-4, 3RD MAIN ROAD,ANNANAGAR (WEST),PIN - 600 PH NO : ALLAHABAD BANK ALLA0211042 600010004 CHENNAI CHENNAI NNAI 102 26263882, EMAIL ID : CHEANNA@CHE .ALLAHABADBA NK.CO.IN MR.ATHIRAMIL AKU K (CHIEF BANGALORE 1540/22,39 E-CROSS,22 MAIN ROAD,4TH T ALLAHABAD BANK ALLA0211819 560010005 CHENNAI CHENNAI MANAGER), MR. JAYANAGAR BLOCK,JAYANAGAR DIST-BANGLAORE,PIN- 560041 SWAINE(SENIOR MANAGER) C N RAVI, CHENNAI 144 GA ROAD,TONDIARPET CHENNAI - 600 081 MURTHY,044- ALLAHABAD BANK ALLA0211881 600010011 CHENNAI CHENNAI TONDIARPET TONDIARPET TAMILNADU 28522093 /28513081 / 28411083 S. SWAMINATHAN CHENNAI V P ,DR. K. ALLAHABAD BANK ALLA0211291 600010008 40/41,MOUNT ROAD,CHENNAI-600002 CHENNAI CHENNAI COLONY TAMINARASAN, 044- 28585641,2854 9262 98, MECRICAR ROAD, R.S.PURAM, COIMBATORE - ALLAHABAD BANK ALLA0210384 641010002 COIIMBATORE COIMBATORE COIMBOTORE 0422 2472333 641002 H1/H2 57 MAIN ROAD, RM COLONY , DINDIGUL- ALLAHABAD BANK ALLA0212319 NON MICR DINDIGUL DINDIGUL DINDIGUL -

BLUE CROSS of INDIA (Recognised by the Animal Welfare Board of India, Govt
BLUE CROSS OF INDIA th 50 ANNUAL REPORT APRIL 1, 2013 to MARCH 31, 2014 www.bluecrossofindia.org BLUE CROSS OF INDIA (Recognised by the Animal Welfare Board of India, Govt. of India) Founders: Capt. V. Sundaram & Mrs. Usha Sundaram The 50th Anniversary of an organisation is not too common an occurrence for most organisations and even rarer for animal welfare groups. This is also an appropriate time to sit down and see what we have really achieved in the last half-century and what we should try to achieve in the next. It is also an even rarer event that five of the original nine founders are still around, with three of them actively involved in the work they began. The other two, in their nineties, are still with us in spirit but even more importantly, the spirit of the four no longer in our presence, guides us in what we do today. Regd. Office : 1, Eldams Road, Chennai – 600 018 Phone: 044-24341778 Hospital & Shelters : Blue Cross Avenue Velachery Road, Guindy, Chennai – 600 032 Phone: 044-22354959 Kunnam Village, Sunkuvarchatram Kanchipuram Dist. Toducadu Village, Sriperumbudur-Tiruvellore Road, Tiruvellore District ABC Centre: Lloyds Colony, Lloyds Road, Chennai – 600 005 Chairman : Mr. Shantilal Pandya Honorary Secretary : Mrs. Saraswathi Haksan Honorary Joint Secretaries : Mr. Sathya Radhakrishnan & Mr. R. Shanker Members Dr. Nanditha Krishna Mrs. Malliga Ravindar Mr. N. Sugal Chand Jain Mr. Suresh Sundaram Mr. L. Nemichand Singhvi Mr. Kantilal Chandak Mr. Prashanth Krishna Mr. Rudra Krishna Mrs. Marion Courtine Mr. P. Mohanakannan Mr. G. Balasubramanian Representative of the AWBI Chairman Emeritus: Dr. -

Post-Tsunami Rehabilitation of Fishing Communities and Fisheries Livelihoods in Tamil Nadu, Kerala and Andhra Pradesh
POST-TSUNAMI REHABILITATION OF FISHING COMMUNITIES AND FISHERIES LIVELIHOODS IN TAMIL NADU, KERALA AND ANDHRA PRADESH BY VENKATESH SALAGRAMA REVISED 12 JANUARY 2006 ICM INTEGRATED COASTAL MANAGEMENT 64-16-3A, PRATAP NAGAR, KAKINADA 533 004 ANDHRA PRADESH INDIA TELE: +91 884 236 4851 EMAIL: [email protected]; [email protected] Post-Tsunami Rehabilitation of Fisheries Livelihoods in India CONTENTS 1. INTRODUCTION....................................................................................................................................2 2. IMPACTS OF TSUNAMI ON COASTAL FISHING COMMUNITIES.............................................................5 Extent of damages..................................................................................................................5 Impacts on craft, gear and infrastructure ...............................................................................5 Impacts of tsunami on different livelihood groups................................................................9 Impact of tsunami on natural resources ...............................................................................10 Impact of tsunami on the psyche of the fishers....................................................................13 Impact of tsunami on social networks .................................................................................13 3. REHABILITATION OF FISHERIES-RELATED LIVELIHOODS.................................................................15 Packages for supporting fisheries-livelihoods -

EXCECUTIVE SUMMARY 1.1. GENERAL the Fisheries
Construction of Fishing Harbour in Kaluveli watersat Azhagankuppam in Villupuram District and at Alamparaikuppam in ChengalpattuDistrict ENVIRONMENTAL IMPACT ASSESSMENT – EXECUTIVE SUMMARY EXCECUTIVE SUMMARY 1.1. GENERAL The Fisheries Department is one of the core line departments of Government of Tamilnadu principally to address the welfare of fishermen community of the state and intended to establish necessary infrastructures required for their safe berthing and hygienic fishing activities. The DoF is committed to have annual budget from Government of Tamilnadu to enhance the contribution of the fishery sector to the food security of the people of Tamilnadu and establish harbor infrastructure facilities at par with International Standard practices for ensuring constant growth in fish catches and its hygienic handling.It also gives top priorities for shore protection and erosion control activities to protect coastal villages from continuous threat from the high waves and cyclones. It works to attract more people into fishing activities;creating more job opportunity among fishery youths; exploring the opportunities in deep sea fishing; doubling the fish production and also to meet the export demands for enhancing the socio-economic status of the fishermen community. Tamilnadu has about1076 km of coastal line that passes through 14 out of 38 districts that consists of four coastal zonesviz.,CoromandelCoast, Palk Bay,GulfofMannarandWestCoast.There are 608 coastal fishermen villages having more than 1.9 Sq.Km of Exclusive Economic Zone (EEZ)